Key Points
-
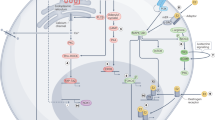
The biological effects of natural and synthetic oestrogen-like ligands are mediated by two oestrogen receptor (ER) subtypes, ERα and ERβ.
-
ERα and ERβ have different physiological roles and thus offer distinct therapeutic opportunities.
-
Treatment with ERβ subtype-selective modulators may retain the benefits of menopausal hormone therapy without its severe adverse side effects, such as breast and endometrial cancer, thromboembolism, stroke and cardiovascular disease.
-
Selective ERβ agonists have shown promising anti-tumorigenic effects in animal models of breast, prostate and colon cancers, and lymphoma.
-
Combination with an ERβ-selective agonist and an ERα-selective antagonist might be an optimal approach for the treatment of hormone-responsive breast cancer.
-
ERβ agonists have shown antihypertensive efficacy and inhibit atherosclerotic lesion development and cardiac hypertrophy in various animal models of heart disease.
-
Selective targeting of ERβ has the potential to be a safe and disease-modifying therapy for multiple sclerosis and Alzheimer's disease, and possibly for other neurodegenerative diseases.
Abstract
The two oestrogen receptor subtypes α and β are hormone-regulated modulators of intracellular signalling and gene expression. Regulation of oestrogen receptor activity is crucial not only for development and homeostasis but also for the treatment of various diseases and symptoms. Classical selective oestrogen receptor modulators are well established in the treatment of breast cancer and osteoporosis, but emerging data suggest that the development of subtype-selective ligands that specifically target either oestrogen receptor-α or oestrogen receptor-β could be a more optimal approach for the treatment of cancer, cardiovascular disease, multiple sclerosis and Alzheimer's disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Boon, W. C., Chow, J. D. & Simpson, E. R. The multiple roles of estrogens and the enzyme aromatase. Prog. Brain Res. 181, 209–232 (2010).
Drummond, A. E. & Fuller, P. J. The importance of ERβ signalling in the ovary. J. Endocrinol. 205, 15–23 (2010).
Taylor, A. H. & Al-Azzawi, F. Immunolocalisation of oestrogen receptor β in human tissues. J. Mol. Endocrinol. 24, 145–155 (2000).
Saji, S. et al. Estrogen receptors α and β in the rodent mammary gland. Proc. Natl Acad. Sci. USA 97, 337–342 (2000).
Rody, A. et al. Methylation of estrogen receptor β promoter correlates with loss of ER-β expression in mammary carcinoma and is an early indication marker in premalignant lesions. Endocr. Relat. Cancer 12, 903–916 (2005).
Yang, X., Li, Y. Z., Mao, Z., Gu, P. & Shang, M. Effects of estrogen and tibolone on bladder histology and estrogen receptors in rats. Chin. Med. J. 122, 381–385 (2009).
Gorres, B. K., Bomhoff, G. L., Gupte, A. A. & Geiger, P. C. Altered estrogen receptor expression in skeletal muscle and adipose tissue of female rats fed a high-fat diet. J. Appl. Physiol. 110, 1046–1053 (2011).
Mitterling, K. L. et al. Cellular and subcellular localization of estrogen and progestin receptor immunoreactivities in the mouse hippocampus. J. Comp. Neurol. 518, 2729–2743 (2010).
Waters, E. M. et al. Estrogen and aging affect the synaptic distribution of estrogen receptor β-immunoreactivity in the CA1 region of female rat hippocampus. Brain Res. 1379, 86–97 (2011).
Rossouw, J. E. et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial. JAMA 288, 321–333 (2002).
Clarkson, T. B. & Appt, S. E. Controversies about HRT—lessons from monkey models. Maturitas 51, 64–74 (2005).
Harman, S. M. et al. Timing and duration of menopausal hormone treatment may affect cardiovascular outcomes. Am. J. Med. 124, 199–205 (2011).
Mendelsohn, M. E. Estrogen actions in the cardiovascular system. Climacteric 12 (Suppl. 1), 18–21 (2009).
Tsai, S. A., Stefanick, M. L. & Stafford, R. S. Trends in menopausal hormone therapy use of US office-based physicians, 2000–2009. Menopause 18, 385–392 (2011).
Santen, R. J. et al. Postmenopausal hormone therapy: an Endocrine Society scientific statement. J. Clin. Endocrinol. Metab. 95, S1–S66 (2010).
Leitman, D. C. et al. Regulation of specific target genes and biological responses by estrogen receptor subtype agonists. Curr. Opin. Pharmacol. 10, 629–636 (2010). This study describes the classification of ERβ-selective agonists based on their potency and efficacy. It discusses the differences between the two ER subtypes with respect to gene regulation and the therapeutic opportunities offered by selective targeting of the two ER subtypes.
Frasor, J. et al. Response-specific and ligand dose-dependent modulation of estrogen receptor (ER) α activity by ERβ in the uterus. Endocrinology 144, 3159–3166 (2003). This report presents data on the different biological responses in the uterus to an ERα-selective agonist and an ERβ-selective agonist.
Helguero, L. A., Faulds, M. H., Gustafsson, J.-Å. & Haldosen, L. A. Estrogen receptors alfa (ERα) and β (ERβ) differentially regulate proliferation and apoptosis of the normal murine mammary epithelial cell line HC11. Oncogene 24, 6605–6616 (2005). This study presents data on the opposing effects of ERα and ERβ on proliferation and apoptosis in the murine mammary cell line HC11.
Gronemeyer, H., Gustafsson, J.-Å. & Laudet, V. Principles for modulation of the nuclear receptor superfamily. Nature Rev. Drug Discov. 3, 950–964 (2004).
Nilsson, S. & Gustafsson, J.-Å. in Nuclear Receptors: Current Concepts and Future Challenges (Proteins and Cell Regulation) (eds Bunce, C. M. & Campbell, M. J.) 91–141 (Springer, 2010).
Powell, E., Wang, Y., Shapiro, D. J. & Xu, W. Differential requirements of Hsp90 and DNA for the formation of estrogen receptor homodimers and heterodimers. J. Biol. Chem. 285, 16125–16134 (2010).
Safe, S. & Kim, K. Non-classical genomic estrogen receptor (ER)/specificity protein and ER/activating protein-1 signaling pathways. J. Mol. Endocrinol. 41, 263–275 (2008).
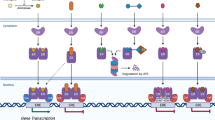
Hall, J. M. & McDonnell, D. P. Coregulators in nuclear estrogen receptor action: from concept to therapeutic targeting. Mol. Interv. 5, 343–357 (2005). A review of co-activator and co-repressor interactions with ER and their importance for the biological responses to agonists, antagonists and SERMs.
Shang, Y., Hu, X., DiRenzo, J., Lazar, M. A. & Brown, M. Cofactor dynamics and sufficiency in estrogen receptor-regulated transcription. Cell 103, 843–852 (2000).
Powell, E. & Xu, W. Intermolecular interactions identify ligand-selective activity of estrogen receptor α/β dimers. Proc. Natl Acad. Sci. USA 105, 19012–19017 (2008).
Monroe, D. G. et al. Estrogen receptor α and β heterodimers exert unique effects on estrogen- and tamoxifen-dependent gene expression in human U2OS osteosarcoma cells. Mol. Endocrinol. 19, 1555–1568 (2005). This study demonstrates that ERα and ERβ heterodimers regulate a unique set of genes, which are distinct from the genes regulated by ERα and ERβ homodimers, in a ligand-dependent fashion.
Charn, T. H. et al. Genome-wide dynamics of chromatin binding of estrogen receptors α and β: mutual restriction and competitive site selection. Mol. Endocrinol. 24, 47–59 (2010).
Perissi, V. & Rosenfeld, M. G. Controlling nuclear receptors: the circular logic of cofactor cycles. Nature Rev. Mol. Cell Biol. 6, 542–554 (2005).
Bramlett, K. S., Wu, Y. & Burris, T. P. Ligands specify coactivator nuclear receptor (NR) box affinity for estrogen receptor subtypes. Mol. Endocrinol. 15, 909–922 (2001).
Gee, A. C., Carlson, K. E., Martini, P. G., Katzenellenbogen, B. S. & Katzenellenbogen, J. A. Coactivator peptides have a differential stabilizing effect on the binding of estrogens and antiestrogens with the estrogen receptor. Mol. Endocrinol. 13, 1912–1923 (1999).
Saijo, K., Collier, J. G., Li, A. C., Katzenellenbogen, J. A. & Glass, C. K. An ADIOL-ERβ-CtBP transrepression pathway negatively regulates microglia-mediated inflammation. Cell 145, 584–595 (2011). Elucidation of an ERβ-mediated transrepressive anti-inflammatory pathway. Evidence is provided that 5-androstene-3β,17β-diol is a physiologically relevant ERβ ligand that triggers ERβ-mediated anti-inflammatory activity by transrepression, whereas the endogenous ER hormone E2 is inactive through this pathway.
Arpino, G., Wiechmann, L., Osborne, C. K. & Schiff, R. Crosstalk between the estrogen receptor and the HER tyrosine kinase receptor family: molecular mechanism and clinical implications for endocrine therapy resistance. Endocr. Rev. 29, 217–233 (2008).
Hammes, S. R. & Levin, E. R. Extranuclear steroid receptors: nature and actions. Endocr. Rev. 28, 726–741 (2007).
Acconcia, F., Ascenzi, P., Fabozzi, G., Visca, P. & Marino, M. S-palmitoylation modulates human estrogen receptor-α functions. Biochem. Biophys. Res. Commun. 316, 878–883 (2004).
Lu, Q. et al. Striatin assembles a membrane signaling complex necessary for rapid, nongenomic activation of endothelial NO synthase by estrogen receptor α. Proc. Natl Acad. Sci. USA 101, 17126–17131 (2004).
Chambliss, K. L. et al. Non-nuclear estrogen receptor α signaling promotes cardiovascular protection but not uterine or breast cancer growth in mice. J. Clin. Invest. 120, 2319–2330 (2010).
Wu, Q., Chambliss, K., Umetani, M., Mineo, C. & Shaul, P. W. Non-nuclear estrogen receptor signaling in endothelium. J. Biol. Chem. 286, 14737–14743 (2011).
Le Romancer, M., Treilleux, I., Bouchekioua-Bouzaghou, K., Sentis, S. & Corbo, L. Methylation, a key step for nongenomic estrogen signaling in breast tumors. Steroids 75, 560–564 (2010).
Madak-Erdogan, Z. et al. Nuclear and extranuclear pathway inputs in the regulation of global gene expression by estrogen receptors. Mol. Endocrinol. 22, 2116–2127 (2008).
Harrington, W. R. et al. Estrogen dendrimer conjugates that preferentially activate extranuclear, nongenomic versus genomic pathways of estrogen action. Mol. Endocrinol. 20, 491–502 (2006).
Amazit, L. et al. Regulation of SRC-3 intercompartmental dynamics by estrogen receptor and phosphorylation. Mol. Cell. Biol. 27, 6913–6932 (2007).
Turjanski, A. G., Vaque, J. P. & Gutkind, J. S. MAP kinases and the control of nuclear events. Oncogene 26, 3240–3253 (2007).
Zhang, L. et al. Estrogen receptor β-selective agonists stimulate calcium oscillations in human and mouse embryonic stem cell-derived neurons. PLoS ONE 5, e11791 (2010).
Zhao, L. & Brinton, R. D. Estrogen receptor α and β differentially regulate intracellular Ca2+ dynamics leading to ERK phosphorylation and estrogen neuroprotection in hippocampal neurons. Brain Res. 1172, 48–59 (2007).
Anstead, G. M., Carlson, K. E. & Katzenellenbogen, J. A. The estradiol pharmacophore: ligand structure-estrogen receptor binding affinity relationships and a model for the receptor binding site. Steroids 62, 268–303 (1997).
Brzozowski, A. M. et al. Molecular basis of agonism and antagonism in the oestrogen receptor. Nature 389, 753–758 (1997). A landmark paper that provided the foundation for the subsequent structure-based design of ER ligands.
Minutolo, F., Macchia, M., Katzenellenbogen, B. S. & Katzenellenbogen, J. A. Estrogen receptor β ligands: recent advances and biomedical applications. Med. Res. Rev. 31, 364–442 (2011). A thorough review of the known chemical classes of ERβ-selective ligands, their pharmacophores and their potential therapeutic applications.
Mohler, M. L. et al. Estrogen receptor β selective nonsteroidal estrogens: seeking clinical indications. Expert Opin. Ther. Pat. 20, 507–534 (2010).
Kuiper, G. G. et al. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors α and β. Endocrinology 138, 863–870 (1997).
Meyers, M. J. et al. Estrogen receptor-β potency-selective ligands: structure-activity relationship studies of diarylpropionitriles and their acetylene and polar analogues. J. Med. Chem. 44, 4230–4251 (2001).
Bruning, J. B. et al. Coupling of receptor conformation and ligand orientation determine graded activity. Nature Chem. Biol. 6, 837–843 (2010).
Paruthiyil, S. et al. Drug and cell type-specific regulation of genes with different classes of estrogen receptor β-selective agonists. PLoS ONE 4, e6271 (2009).
Gellman, S. H. On the role of methionine residues in the sequence-independent recognition of nonpolar protein surfaces. Biochemistry 30, 6633–6636 (1991).
Manas, E. S., Xu, Z. B., Unwalla, R. J. & Somers, W. S. Understanding the selectivity of genistein for human estrogen receptor-β using X-ray crystallography and computational methods. Structure 12, 2197–2207 (2004).
Pike, A. C. et al. Structure of the ligand-binding domain of oestrogen receptor β in the presence of a partial agonist and a full antagonist. EMBO J. 18, 4608–4618 (1999).
Yu, H., Noskov, S. Y. & Roux, B. Two mechanisms of ion selectivity in protein binding sites. Proc. Natl Acad. Sci. USA 107, 20329–20334 (2010).
Hegele-Hartung, C. et al. Impact of isotype-selective estrogen receptor agonists on ovarian function. Proc. Natl Acad. Sci. USA 101, 5129–5134 (2004).
Blizzard, T. A. Selective estrogen receptor modulator medicinal chemistry at Merck. A review. Curr. Top. Med. Chem. 8, 792–812 (2008).
Richardson, T. I. et al. Benzopyrans as selective estrogen receptor β agonists (SERBAs). Part 5: combined A- and C-ring structure-activity relationship studies. Bioorg. Med. Chem. Lett. 17, 5563–5566 (2007).
Veeneman, G. H. & Teerhuis, N. M. Chroman derivatives as estrogenic compounds. US patent 0069303 (2003).
Ferriz, J. M. & Vinsova, J. Prodrug design of phenolic drugs. Curr. Pharm. Des. 16, 2033–2052 (2010).
Wilkening, R. R. et al. Estrogen receptor β-subtype selective tetrahydrofluorenones: use of a fused pyrazole as a phenol bioisostere. Bioorg. Med. Chem. Lett. 16, 3896–3901 (2006).
Gruvberger-Saal, S. K. et al. Estrogen receptor β expression is associated with tamoxifen response in ERα-negative breast carcinoma. Clin. Cancer Res. 13, 1987–1994 (2007).
Hartman, J., Strom, A. & Gustafsson, J. -Å. Estrogen receptor β in breast cancer—diagnostic and therapeutic implications. Steroids 74, 635–641 (2009).
Honma, N. et al. Clinical importance of estrogen receptor-β evaluation in breast cancer patients treated with adjuvant tamoxifen therapy. J. Clin. Oncol. 26, 3727–3734 (2008).
Wu, X. et al. Estrogen receptor-β sensitizes breast cancer cells to the anti-estrogenic actions of endoxifen. Breast Cancer Res. 13, R27 (2011).
Chang, E. C. et al. Estrogen receptors α and β as determinants of gene expression: influence of ligand, dose, and chromatin binding. Mol. Endocrinol. 22, 1032–1043 (2008).
Williams, C., Edvardsson, K., Lewandowski, S. A., Strom, A. & Gustafsson, J.-Å. A genome-wide study of the repressive effects of estrogen receptor β on estrogen receptor α signaling in breast cancer cells. Oncogene 27, 1019–1032 (2008).
Lindberg, K., Helguero, L. A., Omoto, Y., Gustafsson, J.-Å. & Haldosen, L. A. Estrogen receptor β represses Akt signaling in breast cancer cells via downregulation of HER2/HER3 and upregulation of PTEN — implications for tamoxifen sensitivity. Breast Cancer Res. 13, R43 (2011).
Paruthiyil, S. et al. Estrogen receptor β causes a G2 cell cycle arrest by inhibiting CDK1 activity through the regulation of cyclin B1, GADD45A, and BTG2. Breast Cancer Res. Treat. 1 Dec 2010 (doi: 10.1007/s10549-010-1273-5).
Cvoro, A. et al. Selective activation of estrogen receptor-β transcriptional pathways by an herbal extract. Endocrinology 148, 538–547 (2007).
Hartman, J. et al. Estrogen receptor β inhibits angiogenesis and growth of T47D breast cancer xenografts. Cancer Res. 66, 11207–11213 (2006).
Mersereau, J. E. et al. Liquiritigenin is a plant-derived highly selective estrogen receptor β agonist. Mol. Cell. Endocrinol. 283, 49–57 (2008).
Ellem, S. J. & Risbridger, G. P. Aromatase and regulating the estrogen:androgen ratio in the prostate gland. J. Steroid Biochem. Mol. Biol. 118, 246–251 (2010).
McPherson, S. J. et al. Essential role for estrogen receptor β in stromal-epithelial regulation of prostatic hyperplasia. Endocrinology 148, 566–574 (2007).
McPherson, S. J. et al. Estrogen receptor-β activated apoptosis in benign hyperplasia and cancer of the prostate is androgen independent and TNFα mediated. Proc. Natl Acad. Sci. USA 107, 3123–3128 (2010).
Kennelly, R., Kavanagh, D. O., Hogan, A. M. & Winter, D. C. Oestrogen and the colon: potential mechanisms for cancer prevention. Lancet Oncol. 9, 385–391 (2008).
Long, M. D., Martin, C. F., Galanko, J. A. & Sandler, R. S. Hormone replacement therapy, oral contraceptive use, and distal large bowel cancer: a population-based case-control study. Am. J. Gastroenterol. 105, 1843–1850 (2010).
Wada-Hiraike, O. et al. Role of estrogen receptor β in colonic epithelium. Proc. Natl Acad. Sci. USA 103, 2959–2964 (2006).
Hartman, J. et al. Tumor repressive functions of estrogen receptor β in SW480 colon cancer cells. Cancer Res. 69, 6100–6106 (2009).
Weige, C. C., Allred, K. F. & Allred, C. D. Estradiol alters cell growth in nonmalignant colonocytes and reduces the formation of preneoplastic lesions in the colon. Cancer Res. 69, 9118–9124 (2009).
Pinton, G. et al. Estrogen receptor-β affects the prognosis of human malignant mesothelioma. Cancer Res. 69, 4598–4604 (2009).
Pinton, G. et al. Estrogen receptor β exerts tumor repressive functions in human malignant pleural mesothelioma via EGFR inactivation and affects response to gefitinib. PLoS ONE 5, e14110 (2010).
Yakimchuk, K. et al. Effect of ligand-activated estrogen receptor β on lymphoma growth in vitro and in vivo. Leukemia 25, 1103–1110 (2011). This study reports that activation of ERβ with two structurally distinct ERβ-selective agonists suppresses murine T-cell lymphoma tumour growth in vivo by inhibition of proliferation and stimulation of programmed cell death.
Yamasaki, M. et al. Genistein induced apoptotic cell death in adult T-cell leukemia cells through estrogen receptors. Biosci. Biotechnol. Biochem. 74, 2113–2115 (2010).
Arias-Loza, P. A., Jazbutyte, V. & Pelzer, T. Genetic and pharmacologic strategies to determine the function of estrogen receptor α and estrogen receptor β in cardiovascular system. Gend. Med. 5 (Suppl. A), 34–45 (2008).
Vitale, C., Mendelsohn, M. E. & Rosano, G. M. Gender differences in the cardiovascular effect of sex hormones. Nature Rev. Cardiol. 6, 532–542 (2009).
Guo, X., Razandi, M., Pedram, A., Kassab, G. & Levin, E. R. Estrogen induces vascular wall dilation: mediation through kinase signaling to nitric oxide and estrogen receptors α and β. J. Biol. Chem. 280, 19704–19710 (2005).
Zhu, Y. et al. Abnormal vascular function and hypertension in mice deficient in estrogen receptor β. Science 295, 505–508 (2002).
Arias-Loza, P. A. et al. Both estrogen receptor subtypes, α and β, attenuate cardiovascular remodeling in aldosterone salt-treated rats. Hypertension 50, 432–438 (2007).
Jazbutyte, V. et al. Ligand-dependent activation of ERβ lowers blood pressure and attenuates cardiac hypertrophy in ovariectomized spontaneously hypertensive rats. Cardiovasc. Res. 77, 774–781 (2008).
Fliegner, D. et al. Female sex and estrogen receptor-β attenuate cardiac remodeling and apoptosis in pressure overload. Am. J. Physiol. Regul. Integr. Comp. Physiol. 298, R1597–R1606 (2010).
Kararigas, G., Fliegner, D., Gustafsson, J.-Å. & Regitz-Zagrosek, V. The role of the estrogen/estrogen-receptor-β axis in the genomic response to pressure overload-induced hypertrophy. Physiol. Genomics 43, 438–446 (2011).
Pedram, A., Razandi, M., O'Mahony, F., Lubahn, D. & Levin, E. R. Estrogen receptor-β prevents cardiac fibrosis. Mol. Endocrinol. 24, 2152–2165 (2010).
Pelzer, T. et al. Increased mortality and aggravation of heart failure in estrogen receptor-β knockout mice after myocardial infarction. Circulation 111, 1492–1498 (2005).
Skavdahl, M. et al. Estrogen receptor-β mediates male-female differences in the development of pressure overload hypertrophy. Am. J. Physiol. Heart Circ. Physiol. 288, H469–H476 (2005).
Nikolic, I. et al. Treatment with an estrogen receptor-β-selective agonist is cardioprotective. J. Mol. Cell. Cardiol. 42, 769–780 (2007).
Hodgin, J. B. et al. Estrogen receptor α is a major mediator of 17β-estradiol's atheroprotective effects on lesion size in Apoe−/− mice. J. Clin. Invest. 107, 333–340 (2001).
Villablanca, A. C. et al. 17β-estradiol prevents early-stage atherosclerosis in estrogen receptor-α deficient female mice. J. Cardiovasc. Transl. Res. 2, 289–299 (2009).
Pockley, A. G., Georgiades, A., Thulin, T., de Faire, U. & Frostegard, J. Serum heat shock protein 70 levels predict the development of atherosclerosis in subjects with established hypertension. Hypertension 42, 235–238 (2003).
Zhu, J. et al. Increased serum levels of heat shock protein 70 are associated with low risk of coronary artery disease. Arterioscler. Thromb. Vasc. Biol. 23, 1055–1059 (2003).
Martin-Ventura, J. L. et al. Identification by a differential proteomic approach of heat shock protein 27 as a potential marker of atherosclerosis. Circulation 110, 2216–2219 (2004).
Rayner, K. et al. Heat shock protein 27 protects against atherogenesis via an estrogen-dependent mechanism: role of selective estrogen receptor β modulation. Arterioscler. Thromb. Vasc. Biol. 29, 1751–1756 (2009).
Rayner, K. et al. Extracellular release of the atheroprotective heat shock protein 27 is mediated by estrogen and competitively inhibits acLDL binding to scavenger receptor-A. Circ. Res. 103, 133–141 (2008).
Sun, J. et al. Attenuation of atherogenesis via the anti-inflammatory effects of the selective estrogen receptor β modulator 8β-VE2. J. Cardiovasc. Pharmacol. 18 Jun 2011 (doi:10.1097/FJC.0b013e318226bd16).
Gold, S. M. & Voskuhl, R. R. Estrogen and testosterone therapies in multiple sclerosis. Prog. Brain Res. 175, 239–251 (2009).
Soldan, S. S., Alvarez Retuerto, A. I., Sicotte, N. L. & Voskuhl, R. R. Immune modulation in multiple sclerosis patients treated with the pregnancy hormone estriol. J. Immunol. 171, 6267–6274 (2003).
Compston, A. & Coles, A. Multiple sclerosis. Lancet 359, 1221–1231 (2002).
Bodhankar, S., Wang, C., Vandenbark, A. A. & Offner, H. Estrogen-induced protection against experimental autoimmune encephalomyelitis is abrogated in the absence of B cells. Eur. J. Immunol. 41, 1165–1175 (2011).
Crawford, D. K. et al. Oestrogen receptor β ligand: a novel treatment to enhance endogenous functional remyelination. Brain 133, 2999–3016 (2010). This study shows that treatment of EAE mice with the ERβ-selective agonist DPN results in an increased number of differentiated oligodendrocytes and a remarkable remyelination and restoration of axon conduction velocity and axon refractoriness.
Palaszynski, K. M., Liu, H., Loo, K. K. & Voskuhl, R. R. Estriol treatment ameliorates disease in males with experimental autoimmune encephalomyelitis: implications for multiple sclerosis. J. Neuroimmunol. 149, 84–89 (2004).
Sicotte, N. L. et al. Treatment of multiple sclerosis with the pregnancy hormone estriol. Ann. Neurol. 52, 421–428 (2002).
Elloso, M. M., Phiel, K., Henderson, R. A., Harris, H. A. & Adelman, S. J. Suppression of experimental autoimmune encephalomyelitis using estrogen receptor-selective ligands. J. Endocrinol. 185, 243–252 (2005).
Polanczyk, M. et al. Estrogen receptor-1 (Esr1) and -2 (Esr2) regulate the severity of clinical experimental allergic encephalomyelitis in male mice. Am. J. Pathol. 164, 1915–1924 (2004).
Polanczyk, M. et al. The protective effect of 17β-estradiol on experimental autoimmune encephalomyelitis is mediated through estrogen receptor-α. Am. J. Pathol. 163, 1599–1605 (2003).
Tiwari-Woodruff, S., Morales, L. B., Lee, R. & Voskuhl, R. R. Differential neuroprotective and antiinflammatory effects of estrogen receptor (ER)α and ERβ ligand treatment. Proc. Natl Acad. Sci. USA 104, 14813–14818 (2007).
Du, S., Sandoval, F., Trinh, P. & Voskuhl, R. R. Additive effects of combination treatment with anti-inflammatory and neuroprotective agents in experimental autoimmune encephalomyelitis. J. Neuroimmunol. 219, 64–74 (2010).
De Angelis, M., Stossi, F., Carlson, K. A., Katzenellenbogen, B. S. & Katzenellenbogen, J. A. Indazole estrogens: highly selective ligands for the estrogen receptor β. J. Med. Chem. 48, 1132–1144 (2005).
Selkoe, D. J. & Schenk, D. Alzheimer's disease: molecular understanding predicts amyloid-based therapeutics. Annu. Rev. Pharmacol. Toxicol. 43, 545–584 (2003).
Vegeto, E., Benedusi, V. & Maggi, A. Estrogen anti-inflammatory activity in brain: a therapeutic opportunity for menopause and neurodegenerative diseases. Front. Neuroendocrinol. 29, 507–519 (2008).
Pike, C. J., Carroll, J. C., Rosario, E. R. & Barron, A. M. Protective actions of sex steroid hormones in Alzheimer's disease. Front. Neuroendocrinol. 30, 239–258 (2009).
Yue, X. et al. Brain estrogen deficiency accelerates Aβ plaque formation in an Alzheimer's disease animal model. Proc. Natl Acad. Sci. USA 102, 19198–19203 (2005).
Shumaker, S. A. et al. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women's Health Initiative Memory Study. JAMA 291, 2947–2958 (2004).
Brinton, R. D. Investigative models for determining hormone therapy-induced outcomes in brain: evidence in support of a healthy cell bias of estrogen action. Ann. N. Y. Acad. Sci. 1052, 57–74 (2005).
Leissring, M. A. et al. Enhanced proteolysis of β-amyloid in APP transgenic mice prevents plaque formation, secondary pathology, and premature death. Neuron 40, 1087–1093 (2003).
Liang, K. et al. Estrogen stimulates degradation of β-amyloid peptide by up-regulating neprilysin. J. Biol. Chem. 285, 935–942 (2010).
Zhao, L. et al. 17β-Estradiol regulates insulin-degrading enzyme expression via an ERβ/PI3-K pathway in hippocampus: relevance to Alzheimer's prevention. Neurobiol. Aging 6 Jan 2010 (doi: 10.1016/j.neurobiolaging.2009.12.010).
Grady, D. et al. MF101, a selective estrogen receptor β modulator for the treatment of menopausal hot flushes: a phase II clinical trial. Menopause 16, 458–465 (2009).
Zhao, L., Mao, Z. & Brinton, R. D. A select combination of clinically relevant phytoestrogens enhances estrogen receptor β-binding selectivity and neuroprotective activities in vitro and in vivo. Endocrinology 150, 770–783 (2009).
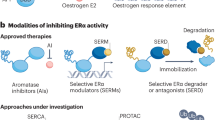
Jordan, V. C. Selective estrogen receptor modulation: a personal perspective. Cancer Res. 61, 5683–5687 (2001). An excellent review of the SERM concept and the clinical applications of SERMs.
Grigsby, J. G. et al. Effects of tamoxifen versus raloxifene on retinal capillary endothelial cell proliferation. J. Ocul. Pharmacol. Ther. 27, 225–233 (2011).
Albertazzi, P. & Sharma, S. Urogenital effects of selective estrogen receptor modulators: a systematic review. Climacteric 8, 214–220 (2005).
Hendrix, S. L. & McNeeley, S. G. Effect of selective estrogen receptor modulators on reproductive tissues other than endometrium. Ann. N. Y. Acad. Sci. 949, 243–250 (2001).
McDonnell, D. P., Clemm, D. L., Hermann, T., Goldman, M. E. & Pike, J. W. Analysis of estrogen receptor function in vitro reveals three distinct classes of antiestrogens. Mol. Endocrinol. 9, 659–669 (1995).
Shang, Y. & Brown, M. Molecular determinants for the tissue specificity of SERMs. Science 295, 2465–2468 (2002).
Tzukerman, M. T. et al. Human estrogen receptor transactivational capacity is determined by both cellular and promoter context and mediated by two functionally distinct intramolecular regions. Mol. Endocrinol. 8, 21–30 (1994).
Shelly, W., Draper, M. W., Krishnan, V., Wong, M. & Jaffe, R. B. Selective estrogen receptor modulators: an update on recent clinical findings. Obstet. Gynecol. Surv. 63, 163–181 (2008).
Barkhem, T. et al. Differential response of estrogen receptor α and estrogen receptor β to partial estrogen agonists/antagonists. Mol. Pharmacol. 54, 105–112 (1998).
Schechter, I. & Berger, A. On the size of the active site in proteases. I. Papain. Biochem. Biophys. Res. Commun. 27, 157–162 (1967).
Acknowledgements
Research in J.-Å.G's laboratory is supported by The Swedish Cancer Fund, the Welch Foundation (Houston, Texas, USA) and the Texas Emerging Technology Fund (Austin, Texas, USA) under agreement number 300-9-1958.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Stefan Nilsson is a Karo Bio employee. Jan-Åke Gustafsson is a consultant with Karo Bio and BioNovo. Konrad F. Koehler is a Karo Bio employee.
Supplementary information
Supplementary information S1 (table)
Phenotypes of ERα- and ERβ-knockout mice (PDF 326 kb)
Supplementary information S2 (table)
Comparison of the physicochemical properties of the pair of amino acid residues that differ between ERα and ERβ in the ligand binding pocket (PDF 231 kb)
Supplementary information S3 (figure)
Interaction energy between ligand and receptor. (PDF 281 kb)
Related links
Glossary
- Detrusor muscle
-
Bladder muscle that relaxes to fill the bladder with urine or contracts to squeeze urine out from the bladder.
- Progestagen
-
Collective name for a ligand of the progesterone receptor, such as progestogen, gestagen or progesterone.
- Palmitoylation
-
The covalent attachment of fatty acids, such as palmitic acid, to cysteine residues on proteins, which facilitates the interaction of the protein with cell membranes.
- Vascular wall hyperplasia
-
Excessive proliferation and growth of cells in the vascular wall.
- Dendrimer
-
A highly branched and roughly spherical polymeric compound that is formed by attachment of monomers to a central core.
- Phyto-oestrogens
-
Plant-derived heterocycles, primarily isoflavones, that mimic the activity of endogenous oestrogens by binding to oestrogen receptors.
- Transrepression
-
The process whereby a regulatory protein — for example, an oestrogen receptor — inhibits or represses the activity of another regulatory protein through protein–protein interaction.
- Oligodendrocytes
-
Branched glia that are involved in neuronal maintenance and support. They produce myelin, which insulates neuronal processes.
- Axon refractoriness
-
A measure of axon responsiveness to stimuli.
Rights and permissions
About this article
Cite this article
Nilsson, S., Koehler, K. & Gustafsson, JÅ. Development of subtype-selective oestrogen receptor-based therapeutics. Nat Rev Drug Discov 10, 778–792 (2011). https://doi.org/10.1038/nrd3551
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrd3551
This article is cited by
-
An overview of PROTACs: a promising drug discovery paradigm
Molecular Biomedicine (2022)
-
Targeted activation of GPER enhances the efficacy of venetoclax by boosting leukemic pyroptosis and CD8+ T cell immune function in acute myeloid leukemia
Cell Death & Disease (2022)
-
Mitochondrial estrogen receptors alter mitochondrial priming and response to endocrine therapy in breast cancer cells
Cell Death Discovery (2021)
-
The peri-menopause in a woman’s life: a systemic inflammatory phase that enables later neurodegenerative disease
Journal of Neuroinflammation (2020)
-
Sirtuins transduce STACs signals through steroid hormone receptors
Scientific Reports (2020)