Key Points
-
The authors propose an interactive, iterative process termed the 'Rosetta Stone approach' for the development of new pharmacotherapies to treat addiction, whereby existing medications are used to validate and improve animal and human laboratory models and then predict viable candidates for new medications.
-
Pharmacotherapies currently on the market for the treatment of addiction have not only highlighted the opportunities for facilitating treatment but are also forming a means for evaluating the development of new pharmacotherapies. Validated animal models of addiction and a surge in understanding of the neurocircuits and neuropharmacological mechanisms involved in the development and maintenance of addiction through basic research have provided numerous viable targets for future medications.
-
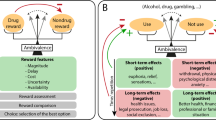
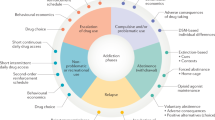
A new focus of basic research into addiction will be on the neuroadaptive changes that account for the transition to dependence and the vulnerability to relapse. The Review places emphasis on several areas to achieve progress in the field: identification of targets by studying neurobiological processes associated with the withdrawal-negative affect ('dark side') stage and the preoccupation-anticipation (craving) stage of the addiction cycle; development of human laboratory studies; pharmacogenetics that could lead to personalized medicine for addiction; understanding and addressing the challenges of streamlining the process of developing investigational new drugs (INDs); and understanding and addressing the challenges unique to clinical trials of pharmacotherapies for addiction.
-
The main impediment is the lack of validated 'decision trees' for deciding which drug candidates should be exploited for INDs.
-
The authors propose that the conceptual framework described in this Review will help promote interactions among National Institute of Health (NIH) institutes and between NIH and private industry to facilitate the creation of such decision trees. The proposed Rosetta Stone approach will therefore not only promote the flourishing of translational research, but also provide a heuristic framework for efficient and effective medications development for addiction.
Abstract
Current pharmacotherapies for addiction represent opportunities for facilitating treatment and are forming a foundation for evaluating new medications. Furthermore, validated animal models of addiction and a surge in understanding of neurocircuitry and neuropharmacological mechanisms involved in the development and maintenance of addiction — such as the neuroadaptive changes that account for the transition to dependence and the vulnerability to relapse — have provided numerous potential therapeutic targets. Here, we emphasize a 'Rosetta Stone approach', whereby existing pharmacotherapies for addiction are used to validate and improve animal and human laboratory models to identify viable new treatment candidates. This approach will promote translational research and provide a heuristic framework for developing efficient and effective pharmacotherapies for addiction.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Koob, G. F. & Le Moal, M. Drug abuse: hedonic homeostatic dysregulation. Science 278, 52–58 (1997).
Kreek, M. J., LaForge, K. S. & Butelman, E. Pharmacotherapy of addictions. Nature Rev. Drug Discov. 1, 710–726 (2002); erratum 1, 926 (2002).
Deroche-Gamonet, V., Belin, D. & Piazza, P. V. Evidence for addiction-like behavior in the rat. Science 305, 1014–1017 (2004).
Vanderschuren, L. J. & Everitt, B. J. Drug seeking becomes compulsive after prolonged cocaine self-administration. Science 305, 1017–1019 (2004).
Heinz, A., Beck, A., Grüsser, S. M., Grace, A. A. & Wrase, J. Identifying the neural circuitry of alcohol craving and relapse vulnerability. Addict. Biol. 14, 108–118 (2009). This study showed a positive correlation between functional brain activation elicited by alcohol-related cues and risk of relapse, suggesting a novel imaging approach in humans.
Sanchis-Segura, C. & Spanagel, R. Behavioural assessment of drug reinforcement and addictive features in rodents: an overview. Addict. Biol. 11, 2–38 (2006).
Koob, G. F. & Le Moal, M. Neurobiology of Addiction Elsevier, London, 2006).
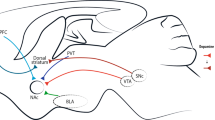
Nestler, E. J. Is there a common molecular pathway for addiction? Nature Neurosci. 8, 1445–1449 (2005). The authors showed that drugs of abuse have very different acute mechanisms of action but converge on the reward pathways of the brain in the ventral tegmental area and nucleus accumbens to produce common functional effects.
Kalivas, P. W. & McFarland, K. Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharmacology 168, 44–56 (2003).
Vanderschuren, L. J. & Everitt, B. J. Behavioral and neural mechanisms of compulsive drug seeking. Eur. J. Pharmacol. 526, 77–88 (2005).
Koob, G. F. & Le Moal, M. Plasticity of reward neurocircuitry and the 'dark side' of drug addiction. Nature Neurosci. 8, 1442–1444 (2005).
Koob, G. F. & Le Moal, M. Addiction and the brain antireward system. Annu. Rev. Psychol. 59, 29–53 (2008). A neurobiological model of the brain emotional systems was proposed to explain the persistent changes in motivation that are associated with vulnerability to dependence in addiction.
Koob, G. F. A role for brain stress systems in addiction. Neuron 59, 11–34 (2008).
Nestler, E. J. Molecular neurobiology of addiction. Am. J. Addict. 10, 201–217 (2001).
Shaham, Y., Shalev, U., Lu, L., de Wit, H. & Stewart, J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology 168, 3–20 (2003). This review elegantly summarizes the neuronal events that mediate reinstatement of heroin-, cocaine- and alcohol-seeking following acute priming injections of drugs, drug-associated cues and environmental stressors.
Schultz, W. Multiple dopamine functions at different time courses. Annu. Rev. Neurosci. 30, 259–288 (2007).
Berridge, K. C. The debate over dopamine's role in reward: the case for incentive salience. Psychopharmacology 191, 391–431 (2007).
Weiss, F. & Koob, G. F. Drug addiction: functional neurotoxicity of the brain reward systems. Neurotox. Res. 3, 145–156 (2000).
Weiss, F., Markou, A., Lorang, M. T. & Koob, G. F. Basal extracellular dopamine levels in the nucleus accumbens are decreased during cocaine withdrawal after unlimited-access self-administration. Brain Res. 593, 314–318 (1992).
Weiss, F. et al. Ethanol self-administration restores withdrawal-associated deficiencies in accumbal dopamine and 5-hydroxytryptamine release in dependent rats. J. Neurosci. 16, 3474–3485 (1996).
Melis, M., Spiga, S. & Diana, M. The dopamine hypothesis of drug addiction: hypodopaminergic state. Int. Rev. Neurobiol. 63, 101–154 (2005). The authors report that tonic mesolimbic dopamine transmission seems to be drastically reduced in animal models of drug addiction and human subjects with the disease, and suggest that restoring dopamine transmission (not necessarily with classic receptor-oriented drugs) may reveal new treatment options.
Pulvirenti, L. & Koob, G. F. Being partial to psychostimulant addiction therapy. Trends Pharmacol. Sci. 23, 151–153 (2002).
Clark, D. et al. Behavioural profile of partial D2 dopamine receptor agonists: 1. Atypical inhibition of d-amphetamine-induced locomotor hyperactivity and stereotypy. Psychopharmacology 105, 381–392 (1991).
Pulvirenti, L. & Koob, G. F. Dopamine receptor agonists, partial agonists and psychostimulant addiction. Trends Pharmacol. Sci. 15, 374–379 (1994).
Svensson, K. et al. Effects of the partial dopamine receptor agonists SDZ 208-911, SDZ 208-912 and terguride on central monoamine receptors: a behavioral, biochemical and electrophysiological study. Naunyn Schmiedebergs Arch. Pharmacol. 344, 263–274 (1991).
Pulvirenti, L., Smith, D. & Koob, G. F. SDZ 208-911, an amino-ergoline with partial dopamine agonistic properties, dose dependently increases cocaine self-administration in the rat. Psychopharmacology 113, 518–520 (1994).
Pulvirenti, L., Balducci, C., Piercy, M. & Koob, G. F. Characterization of the effects of the partial dopamine agonist terguride on cocaine self-administration in the rat. J. Pharmacol. Exp. Ther. 286, 1231–1238 (1998).
Izzo, E., Orsini, C., Koob, G. F. & Pulvirenti, L. A dopamine partial agonist and antagonist block amphetamine self-administration in a progressive ratio schedule. Pharmacol. Biochem. Behav. 68, 701–708 (2001).
Bono, G., Balducci, C., Richelmi, P., Koob, G. F. & Pulvirenti, L. Dopamine partial receptor agonists reduce ethanol intake in the rat. Eur. J. Pharmacol. 296, 233–238 (1996).
Orsini, C., Koob, G. F. & Pulvirenti, L. Dopamine partial agonist reverses amphetamine withdrawal in rats. Neuropsychopharmacology 25, 789–792 (2001).
Hoefer, M. E., Voskanian, S. J., Koob, G. F. & Pulvirenti, L. Effects of terguride, ropinirole, and acetyl-L-carnitine on methamphetamine withdrawal in the rat. Pharmacol. Biochem. Behav. 83, 403–409 (2006).
Wee, S., Wang, Z., Woolverton, W. L., Pulvirenti, L. & Koob, G. F. Effect of aripiprazole, a partial D2 receptor agonist, on increased rate of methamphetamine self-administration in rats with prolonged access. Neuropsychopharmacology 32, 2238–2247 (2007).
Heidbreder, C. A. et al. The role of central dopamine D3 receptors in drug addiction: a review of pharmacological evidence. Brain Res. Rev. 49, 77–105 (2005).
Gyertyan, I. et al. Effects of RGH-237 (N-(4-(4-(3-aminocarbonyl-phenyl)-piperazin-1-yl)-butyl)-4-bromo-benzamide), an orally active, selective dopamine D3 receptor partial agonist in animal models of cocaine abuse. J. Pharmacol. Exp. Ther. 320, 1268–1278 (2007).
Pilla, M. et al. Selective inhibition of cocaine-seeking behaviour by a partial dopamine D3 receptor agonist. Nature 400, 371–375 (1999).
Aujla, H. & Beninger, R. J. The dopamine D3 receptor-preferring partial agonist BP 897 dose-dependently attenuates the expression of amphetamine-conditioned place preference in rats. Behav. Pharmacol. 16, 181–186 (2005).
Caine, S. B. & Koob, G. F. Effects of mesolimbic dopamine depletion on responding maintained by cocaine and food. J. Exp. Anal. Behav. 61, 213–221 (1994).
Haile, C. N. & Kosten, T. A. Differential effects of D1- and D2-like compounds on cocaine self-administration in Lewis and Fischer 344 inbred rats. J. Pharmacol. Exp. Ther. 299, 509–518 (2001).
Spanagel, R. & Kiefer, F. Drugs for relapse prevention of alcoholism: ten years of progress. Trends Pharmacol. Sci. 29, 109–115 (2008). This review summarizes the many neurochemical pathways involved in mediating craving and relapse to alcohol, and focuses on new targets in these domains for the treatment of alcoholism.
Rassnick, S., D'Amico, E., Riley, E. & Koob, G. F. GABA antagonist and benzodiazepine partial inverse agonist reduce motivated responding for ethanol. Alcohol. Clin. Exp. Res. 17, 124–130 (1993).
Hyytia, P. & Koob, G. F. GABAA receptor antagonism in the extended amygdala decreases ethanol self-administration in rats. Eur. J. Pharmacol. 283, 151–159 (1995).
Weerts, E. M., Froestl, W. & Griffiths, R. R. Effects of GABAergic modulators on food and cocaine self-administration in baboons. Drug Alcohol Depend. 80, 369–376 (2005).
Paterson, N. E. et al. Positive modulation of GABAB receptors decreased nicotine self-administration and counteracted nicotine-induced enhancement of brain reward function in rats. J. Pharmacol. Exp. Ther. 326, 306–314 (2008).
Colombo, G. et al. Baclofen suppresses motivation to consume alcohol in rats. Psychopharmacology 167, 221–224 (2003).
Xi, Z. X. & Stein, E. A. Baclofen inhibits heroin self-administration behavior and mesolimbic dopamine release. J. Pharmacol. Exp. Ther. 290, 1369–1374 (1999).
Frye, G. D., McCown, T. J. & Breese, G. R. Differential sensitivity of ethanol withdrawal signs in the rat to gamma-aminobutyric acid (GABA) mimetics: blockade of audiogenic seizures but not forelimb tremors. J. Pharmacol. Exp. Ther. 226, 720–725 (1983).
Addolorato, G. et al. Baclofen efficacy in reducing alcohol craving and intake: a preliminary double-blind randomized controlled study. Alcohol Alcohol. 37, 504–508 (2002).
Addolorato, G. et al. Rapid suppression of alcohol withdrawal syndrome by baclofen. Am. J. Med. 112, 226–229 (2002).
Walker, B. M. & Koob, G. F. The γ-aminobutyric acid-B receptor agonist baclofen attenuates responding for ethanol in ethanol-dependent rats. Alcohol. Clin. Exp. Res. 31, 11–18 (2007).
Ahmadi-Abhari, S. A. et al. Baclofen versus clonidine in the treatment of opiates withdrawal, side-effects aspect: a double-blind randomized controlled trial. J. Clin. Pharmacol. Ther. 26, 67–71 (2001).
Sills, G. J. The mechanisms of action of gabapentin and pregabalin. Curr. Opin. Pharmacol. 6, 108–113 (2006).
Taylor, C. P. et al. A summary of mechanistic hypotheses of gabapentin pharmacology. Epilepsy Res. 29, 233–249 (1998).
Gotz, E., Feuerstein, T. J., Lais, A. & Meyer, D. K. Effects of gabapentin on release of gamma-aminobutyric acid from slices of rat neostriatum. Arzneimittelforschung 43, 636–638 (1993).
Fink, K., Meder, W., Dooley, D. J. & Göthert, M. Inhibition of neuronal Ca2+ influx by gabapentin and subsequent reduction of neurotransmitter release from rat neocortical slices. Brit. J. Pharmacol. 130, 900–906 (2000).
Bertrand, S. et al. The anticonvulsant, antihyperalgesic agent gabapentin is an agonist at brain γ-aminobutyric acid type B receptors negatively coupled to voltage-dependent calcium channels. J. Pharmacol. Exp. Ther. 298, 15–24 (2001).
Roberto, M. et al. Cellular and behavioral interactions of gabapentin with alcohol dependence. J. Neurosci. 28, 5762–5771 (2008).
Mason, B. J., Light, J. M., Williams, L. D. & Drobes, D. J. Proof-of-concept human laboratory study for protracted abstinence in alcohol dependence: effects of gabapentin. Addict. Biol. 14, 73–83 (2009).
Koob, G. F., Heinrichs, S. C., Menzaghi, F., Pich, E. M. & Britton, K. T. Corticotropin releasing factor, stress and behavior. Semin. Neurosci. 6, 221–229 (1994).
Swanson, L. W., Sawchenko, P. E., Rivier, J. & Vale, W. The organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology 36, 165–186 (1983).
Sarnyai, Z. et al. Brain corticotropin-releasing factor mediates “anxiety-like” behavior induced by cocaine withdrawal in rats. Brain Res. 675, 89–97 (1995).
Basso, A. M., Spina, M., Rivier, J., Vale, W. & Koob, G. F. Corticotropin-releasing factor antagonist attenuates the “anxiogenic-like” effect in the defensive burying paradigm but not in the elevated plus-maze following chronic cocaine in rats. Psychopharmacology 145, 21–30 (1999).
Knapp, D. J., Overstreet, D. H., Moy, S. S. & Breese, G. R. SB242084, flumazenil, and CRA1000 block ethanol withdrawal-induced anxiety in rats. Alcohol 32, 101–111 (2004).
Overstreet, D. H., Knapp, D. J. & Breese, G. R. Modulation of multiple ethanol withdrawal-induced anxiety-like behavior by CRF and CRF1 receptors. Pharmacol. Biochem. Behav. 77, 405–413 (2004).
George, O. et al. CRF–CRF1 system activation mediates withdrawal-induced increases in nicotine self-administration in nicotine-dependent rats. Proc. Natl Acad. Sci. USA 104, 17198–17203 (2007).
Stinus, L., Cador, M., Zorrilla, E. P. & Koob, G. F. Buprenorphine and a CRF1 antagonist block the acquisition of opiate withdrawal-induced conditioned place aversion in rats. Neuropsychopharmacology 30, 90–98 (2005).
Rodriguez de Fonseca, F., Carrera, M. R. A., Navarro, M., Koob, G. F. & Weiss, F. Activation of corticotropin-releasing factor in the limbic system during cannabinoid withdrawal. Science, 276, 2050–2054 (1997).
Funk, C. K., O'Dell, L. E., Crawford, E. F. & Koob, G. F. Corticotropin-releasing factor within the central nucleus of the amygdala mediates enhanced ethanol self-administration in withdrawn, ethanol-dependent rats. J. Neurosci. 26, 11324–11332 (2006).
Merlo-Pich, E. et al. Increase of extracellular corticotropin-releasing factor-like immunoreactivity levels in the amygdala of awake rats during restraint stress and ethanol withdrawal as measured by microdialysis. J. Neurosci. 15, 5439–5447 (1995).
Olive, M. F., Koenig, H. N., Nannini, M. A. & Hodge, C. W. Elevated extracellular CRF levels in the bed nucleus of the stria terminalis during ethanol withdrawal and reduction by subsequent ethanol intake. Pharmacol. Biochem. Behav. 72, 213–220 (2002).
Richter, R. M. & Weiss, F. In vivo CRF release in rat amygdala is increased during cocaine withdrawal in self-administering rats. Synapse 32, 254–261 (1999).
Weiss, F. et al. Compulsive drug-seeking behavior and relapse: neuroadaptation, stress, and conditioning factors. Ann. NY Acad. Sci. 937, 1–26 (2001).
Baldwin, H. A., Rassnick, S., Rivier, J., Koob, G. F. & Britton, K. T. CRF antagonist reverses the “anxiogenic” response to ethanol withdrawal in the rat. Psychopharmacology 103, 227–232 (1991).
Valdez, G. R. et al. Increased ethanol self-administration and anxiety-like behavior during acute withdrawal and protracted abstinence: regulation by corticotropin-releasing factor. Alcohol. Clin. Exp. Res. 26, 1494–1501 (2002).
Gehlert, D. R. et al. 3-(4-chloro-2-morpholin-4-yl-thiazol-5-yl)-8-(1-ethylpropyl)-2,6-dimethyl-imidazo(1,2-b)pyridazine: a novel brain-penetrant, orally available corticotropin-releasing factor receptor 1 antagonist with efficacy in animal models of alcoholism. J. Neurosci. 27, 2718–2726 (2007).
Funk, C. K., Zorrilla, E. P., Lee, M. J., Rice, K. C. & Koob, G. F. Corticotropin-releasing factor 1 antagonists selectively reduce ethanol self-administration in ethanol-dependent rats. Biol. Psychol. 61, 78–86 (2007). This study showed that CRF 1 receptors play an important part in mediating excessive alcohol self-administration in dependent rats, but not in non-dependent rats, and that CRF 1 receptor antagonists could be new pharmacotherapeutic targets for the treatment of alcoholism in humans.
Specio, S. E. et al. CRF1 receptor antagonists attenuate escalated cocaine self-administration in rats. Psychopharmacology 196, 473–482 (2008).
Greenwell, T. N. et al. Corticotropin-releasing factor-1 receptor antagonists decrease heroin self-administration in long-, but not short-access rats. Addict. Biol. 14, 130–143 (2009).
Zobel, A. W. et al. Effects of the high-affinity corticotropin-releasing hormone receptor 1 antagonist R121919 in major depression: the first 20 patients treated. J. Psychiatr. Res. 34, 171–181 (2000).
Binneman, B. et al. A 6-week randomized, placebo-controlled trial of CP-316311 (a selective CRH1 antagonist) in the treatment of major depression. Am. J. Psychiatry 165, 617–620 (2008).
Walker, B. M., Rasmussen, D. D., Raskind, M. A. & Koob, G. F. α1-Noradrenergic receptor antagonism blocks dependence-induced increases in responding for ethanol. Alcohol 42, 91–97 (2008).
Greenwell, T. N., Walker, B. M., Cottone, P., Zorrilla, E. P. & Koob, G. F. The α1 adrenergic receptor antagonist prazosin reduces heroin self-administration in rats with extended access to heroin administration. Pharmacol. Biochem. Behav. 91, 295–302 (2009).
Wee, S., Mandyam, C. D., Lekic, D. M. & Koob, G. F. α1-Noradrenergic system role in increased motivation for cocaine intake in rats with prolonged access. Eur. Neuropsychopharmacol. 18, 303–311 (2008).
Shippenberg, T. S., Zapata, A. & Chefer, V. I. Dynorphin and the pathophysiology of drug addiction. Pharmacol. Ther. 116, 306–321 (2007).
Mucha, R. F. & Herz, A. Motivational properties of kappa and mu opioid receptor agonists studied with place and taste preference conditioning. Psychopharmacology 86, 274–280 (1985).
Pfeiffer, A., Brantl, V., Herz, A. & Emrich, H. M. Psychotomimesis mediated by kappa opiate receptors. Science 233, 774–776 (1986).
Sivam, S. P. Cocaine selectively increases striatonigral dynorphin levels by a dopaminergic mechanism. J. Pharmacol. Exp. Ther. 250, 818–824 (1989).
Spangler, R., Unterwald, E. M. & Kreek, M. J. “Binge” cocaine administration induces a sustained increase of prodynorphin mRNA in rat caudate-putamen. Mol. Brain Res. 19, 323–327 (1993). This study showed a substantial increase in the concentration of prodynorphin mRNA in caudate putamen extracts of rats injected with cocaine, using a binge administration pattern designed to mimic cocaine abuse in humans.
Hurd, Y. L. & Herkenham, M. Molecular alterations in the neostriatum of human cocaine addicts. Synapse 13, 357–369 (1993).
Unterwald, E. M., Rubenfeld, J. M. & Kreek, M. J. Repeated cocaine administration upregulates kappa and mu, but not delta, opioid receptors. Neuroreport 5, 1613–1616 (1994).
Nestler, E. J. Historical review: molecular and cellular mechanisms of opiate and cocaine addiction. Trends Pharmacol. Sci. 25, 210–218 (2004).
Shirayama, Y. et al. Stress increases dynorphin immunoreactivity in limbic brain regions and dynorphin antagonism produces antidepressant-like effects. J. Neurochem. 90, 1258–1268 (2004).
Land, B. B. et al. The dysphoric component of stress is encoded by activation of the dynorphin κ-opioid system. J. Neurosci. 28, 407–414 (2008).
McLaughlin, J. P., Marton-Popovici, M. & Chavkin, C. Kappa opioid receptor antagonism and prodynorphin gene disruption block stress-induced behavioral responses. J. Neurosci. 23, 5674–5683 (2003).
Beardsley, P. M., Howard, J. L., Shelton, K. L. & Carroll, F. I. Differential effects of the novel kappa opioid receptor antagonist, JDTic, on reinstatement of cocaine-seeking induced by footshock stressors vs cocaine primes and its antidepressant-like effects in rats. Psychopharmacology 183, 118–126 (2005).
Valdez, G. R., Platt, D. M., Rowlett, J. K., Rüedi-Bettschen, D. & Spealman, R. D. Kappa agonist-induced reinstatement of cocaine seeking in squirrel monkeys: a role for opioid and stress-related mechanisms. J. Pharmacol. Exp. Ther. 323, 525–533 (2007).
Negus, S. S. et al. Effect of antagonists selective for mu, delta and kappa opioid receptors on the reinforcing effects of heroin in rats. J. Pharmacol. Exp. Ther. 265, 1245–1252 (1993).
Walker, B. M. & Koob, G. F. Pharmacological evidence for a motivational role of κ-opioid systems in ethanol dependence. Neuropsychopharmacology 33, 643–652 (2008).
Heilig, M., Koob, G. F., Ekman, R. & Britton, K. T. Corticotropin-releasing factor and neuropeptide Y: role in emotional integration. Trends Neurosci. 17, 80–85 (1994).
Ciccocioppo, R., Angeletti, S., Panocka, I. & Massi, M. Nociceptin–orphanin FQ and drugs of abuse. Peptides 21, 1071–1080 (2000).
George, D. T. et al. Neurokinin 1 receptor antagonism as a possible therapy for alcoholism. Science 319, 1536–1539 (2008).
Hoffman, P. L., Rabe, C. S., Moses, F. & Tabakoff, B. N-methyl-D-aspartate receptors and ethanol: inhibition of calcium flux and cyclic GMP production. J. Neurochem. 52, 1937–1940 (1989).
de Witte, P., Littleton, J., Parot, P. & Koob, G. Neuroprotective and abstinence-promoting effects of acamprosate: elucidating the mechanism of action. CNS Drugs 19, 517–537 (2005).
Littleton, J. M. Acamprosate in alcohol dependence: implications of a unique mechanism of action. J. Addict. Med. 1, 115–125 (2007).
Li, Y., Vartanian, A. J., White, F. J., Xue, C. J. & Wolf, M. E. Effects of the AMPA receptor antagonist NBQX on the development and expression of behavioral sensitization to cocaine and amphetamine. Psychopharmacology 134, 266–276 (1997).
Ungless, M. A., Whistler, J. L., Malenka, R. C. & Bonci, A. Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature 411, 583–387 (2001).
Conrad, K. L. et al. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature 454, 118–121 (2008). This paper proposed that increased levels of AMPA receptors lacking the GluR2 subunit causes increased reactivity of nucleus accumbens neurons to cocaine-related cues and leads to an intensification of drug craving and relapse.
Kalivas, P. W. et al. Glutamate transmission and addiction to cocaine. Ann. NY Acad. Sci. 1003, 169–175 (2003).
Everitt, B. J. & Wolf, M. E. Psychomotor stimulant addiction: a neural systems perspective. J. Neurosci. 22, 3312–3320 (2002); erratum 22, 1a (2002).
Vorel, S. R., Liu, X., Hayes, R. J., Spector, J. A. & Gardner, E. L. Relapse to cocaine-seeking after hippocampal theta burst stimulation. Science 292, 1175–1178 (2001).
Engblom, D. et al. Glutamate receptors on dopamine neurons control the persistence of cocaine seeking. Neuron 59, 497–508 (2008).
Stringer, S., Rueve, M. & Mossman, D. Topiramate as treatment for alcohol dependence. J. Am. Med. Assoc. 299, 405–406 (2008).
Olmsted, C. L. & Kockler, D. R. Topiramate for alcohol dependence. Ann. Pharmacother. 42, 1475–1480 (2008).
Gabriel, K. I. & Cunningham, C. L. Effects of topiramate on ethanol and saccharin consumption and preferences in C57BL/6J mice. Alcohol. Clin. Exp. Res. 29, 75–80 (2005).
Farook, J. M., Lewis, B., Littleton, J. M. & Barron, S. Topiramate attenuates the stress-induced increase in alcohol consumption and preference in male C57BL/6J mice. Physiol. Behav. 96, 189–193 (2009).
Gremel, C. M., Gabriel, K. I. & Cunningham, C. L. Topiramate does not affect the acquisition or expression of ethanol conditioned place preference in DBA/2J or C57BL/6J mice. Alcohol. Clin. Exp. Res. 30, 783–790 (2006).
Di Ciano, P. & Everitt, B. J. Dissociable effects of antagonism of NMDA and AMPA/KA receptors in the nucleus accumbens core and shell on cocaine-seeking behavior. Neuropsychopharmacology 25, 341–360 (2001).
Zhao, Y. et al. Activation of group II metabotropic glutamate receptors attenuates both stress and cue-induced ethanol-seeking and modulates c-fos expression in the hippocampus and amygdala. J. Neurosci. 26, 9967–9974 (2006).
Schroeder, J. P. et al. Cue-induced reinstatement of alcohol-seeking behavior is associated with increased ERK1/2 phosphorylation in specific limbic brain regions: blockade by the mGluR5 antagonist MPEP. Neuropharmacology 55, 546–554 (2008).
Backstrom, P. & Hyytia, P. Ionotropic and metabotropic glutamate receptor antagonism attenuates cue-induced cocaine seeking. Neuropsychopharmacology 31, 778–786 (2006).
Haney, M. Self-administration of cocaine, cannabis and heroin in the human laboratory: benefits and pitfalls. Addict. Biol. 14, 9–21 (2009).
Mello, N. K., Mendelson, J. H., Kuehnle, J. C. & Sellers, M. S. Operant analysis of human heroin self-administration and the effects of naltrexone. J. Pharmacol. Exp. Ther. 216, 45–54 (1981).
Comer, S. D., Collins, E. D. & Fischman, M. W. Choice between money and intranasal heroin in morphine-maintained humans. Behav. Pharmacol. 8, 677–690 (1997).
Vocci, F. J. & Elkashef, A. Pharmacotherapy and other treatments for cocaine abuse and dependence. Curr. Opin. Psychiatry 18, 265–270 (2005).
Logan, G. D. in Inhibitory Processes in Attention, Memory and Language (eds Dagenbach, D. & Carr, T. H.) 189–236 (Academic Press, San Diego, 1994).
Dole, V. P. Implications of methadone maintenance for theories of narcotic addiction. J. Am. Med. Assoc. 260, 3025–3029 (1988).
Haney, M. et al. Marijuana withdrawal in humans: effects of oral THC or divalproex. Neuropsychopharmacology 29, 158–170 (2004).
Evans, D. E. & Drobes, D. J. Nicotine self-medication of cognitive-attentional processing. Addict. Biol. 14, 32–42 (2009).
Kassel, J. D. & Unrod, M. Smoking, anxiety, and attention: support for the role of nicotine in attentionally mediated anxiolysis. J. Abnorm. Psychol. 109, 161–166 (2000).
Koob, G. F. & Kreek, M. J. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am. J. Psychiatry 164, 1149–1159 (2007).
Drobes, D. J., Anton, R. F., Thomas, S. E. & Voronin, K. A clinical laboratory paradigm for evaluating medication effects on alcohol consumption: naltrexone and nalmefene. Neuropsychopharmacology 28, 755–764 (2003).
Krishnan-Sarin, S., Krystal, J. H., Shi, J., Pittman, B. & O'Malley, S. S. Family history of alcoholism influences naltrexone-induced reduction in alcohol drinking. Biol. Psychiatry 62, 694–697 (2007).
Chornock, W. M., Stitzer, M. L., Gross, J. & Leischow, S. Experimental model of smoking re-exposure: effects on relapse. Psychopharmacology 108, 495–500 (1992).
Carter, B. L. & Tiffany, S. T. Meta-analysis of cue-reactivity in addiction research. Addiction 94, 327–340 (1999).
Monti, P. M. et al. Naltrexone's effect on cue-elicited craving among alcoholics in treatment. Alcohol. Clin. Exp. Res. 23, 1386–1394 (1999).
Cooney, N. L., Litt, M. D., Morse, P. A., Bauer, L. O. & Gaupp, L. Alcohol cue reactivity, negative-mood reactivity, and relapse in treated alcoholic men. J. Abnorm. Psychol. 106, 243–250 (1997).
Miranda, R. Jr et al. Effects of topiramate on urge to drink and the subjective effects of alcohol: a preliminary laboratory study. Alcohol. Clin. Exp. Res. 32, 489–497 (2008).
Shiffman, S. et al. Efficacy of acute administration of nicotine gum in relief of cue-provoked cigarette craving. Psychopharmacology 166, 343–350 (2003).
Hersh, D., Bauer, L. O. & Kranzler, H. R. Carbamazepine and cocaine-cue reactivity. Drug Alcohol Depend. 39, 213–221 (1995).
Robbins, S. J., Ehrman, R. N., Childress, A. R. & O'Brien, C. P. Using cue reactivity to screen medications for cocaine abuse: a test of amantadine hydrochloride. Addict. Behav. 17, 491–499 (1992).
Marlatt, G. & Gordon, J. in Behavioral Medicine: Changing Health Lifestyles (eds Davidson, P. & Davidson, S.) 410–452 (Brunner/Mazel, New York, 1980).
Childress, A. R. et al. Can induced moods trigger drug-related responses in opiate abuse patients? J. Subst. Abuse Treat. 11, 17–23 (1994).
Sinha, R., Fuse, T., Aubin, L. R. & O'Malley, S. S. Psychological stress, drug-related cues and cocaine craving. Psychopharmacology 152, 140–148 (2000).
Lang, P. J., Kozak, M. J., Miller, G. A., Levin, D. N. & McLean, A. Jr. Emotional imagery: conceptual structure and pattern of somato-visceral response. Psychophysiology 17, 179–192 (1980).
Fox, H. C., Bergquist, K. L., Hong, K. I. & Sinha, R. Stress-induced and alcohol cue-induced craving in recently abstinent alcohol-dependent individuals. Alcohol. Clin. Exp. Res. 31, 395–403 (2007).
Sinha, R., Garcia, M., Paliwal, P., Kreek, M. J. & Rounsaville, B. J. Stress-induced cocaine craving and hypothalamic-pituitary-adrenal responses are predictive of cocaine relapse outcomes. Arch. Gen. Psychiatry 63, 324–331 (2006).
Breese, G. R., Overstreet, D. H., Knapp, D. J. & Navarro, M. Prior multiple ethanol withdrawals enhance stress-induced anxiety-like behavior: inhibition by CRF1- and benzodiazepine-receptor antagonists and a 5-HT1a-receptor agonist. Neuropsychopharmacology 30, 1662–1669 (2005).
al'Absi, M., Hatsukami, D. & Davis, G. L. Attenuated adrenocorticotropic responses to psychological stress are associated with early smoking relapse. Psychopharmacology 181, 107–117 (2005).
Sinha, R., Kimmerling, A., Doebrick, C. & Kosten, T. R. Effects of lofexidine on stress-induced and cue-induced opioid craving and opioid abstinence rates: preliminary findings. Psychopharmacology 190, 569–574 (2007).
Mason, B. J., Light, J. M., Escher, T. & Drobes, D. J. Effect of positive and negative affective stimuli and beverage cues on measures of craving in non treatment-seeking alcoholics. Psychopharmacology 200, 141–150 (2008).
McKee, S. A. Developing human laboratory models of smoking lapse behavior for medication screening. Addict. Biol. 14, 99–107 (2009).
Braus, D. F. et al. Alcohol-associated stimuli activate the ventral striatum in abstinent alcoholics. J. Neural Transm. 108, 887–894 (2001).
Grusser, S. M. et al. Cue-induced activation of the striatum and medial prefrontal cortex is associated with subsequent relapse in abstinent alcoholics. Psychopharmacology 175, 296–302 (2004).
Wrase, J. et al. Dysfunction of reward processing correlates with alcohol craving in detoxified alcoholics. Neuroimage 35, 787–794 (2007).
Bond, C. et al. Single-nucleotide polymorphism in the human mu opioid receptor gene alters β-endorphin binding and activity: possible implications for opiate addiction. Proc. Natl Acad. Sci. USA 95, 9608–9613 (1998).
Treutlein, J. et al. Genetic association of the human corticotropin releasing hormone receptor 1 (CRHR1) with binge drinking and alcohol intake patterns in two independent samples. Mol. Psychiatry 11, 594–602 (2006).
Garbutt, J. C. et al. Efficacy and tolerability of long-acting injectable naltrexone for alcohol dependence: a randomized controlled trial. J. Am. Med. Assoc. 293, 1617–1625 (2005); errata 293, 1978, 2864 (2005).
Huestis, M. A. & Cone, E. J. Differentiating new marijuana use from residual drug excretion in occasional marijuana users. J. Anal. Toxicol. 22, 445–454 (1998).
Sobell, L. C. & Sobell, M. B. in Measuring Alcohol Consumption: Psychosocial and Biochemical Methods (eds Litten, R. Z. & Allen, J. P.) 41–72 (Human Press, Totowa, 1992).
Office of National Drug Control Policy — The Economic Costs of Drug Abuse in the United States: 1992–2002. (Office of National Drug Control Policy, Washington DC, 2004).
National Institute on Alcohol Abuse and Alcoholism 10th special report to the US Congress on alcohol and health: highlights from current research. (National Institute on Alcohol Abuse and Alcoholism, Bethesda, 2000).
Centers for Disease Control and Prevention — annual smoking-attributable mortality, years of potential life lost, and productivity losses: United States, 1997–2001. Morb. Mort. Wkly Rep. 54, 625–628 (2005).
O'Brien, C. P. & McLellan, A. T. Myths about the treatment of addiction. Lancet 347, 237–240 (1996).
Lotsch, J. & Geisslinger, G. Are μ-opioid receptor polymorphisms important for clinical opioid therapy? Trends Mol. Med. 11, 82–89 (2005).
Ray, L. A. & Hutchison, K. E. A polymorphism of the mu-opioid receptor gene (OPRM1) and sensitivity to the effects of alcohol in humans. Alcohol. Clin. Exp. Res. 28, 1789–1795 (2004).
Anton, R. F. et al. An evaluation of μ-opioid receptor (OPRM1) as a predictor of naltrexone response in the treatment of alcohol dependence: results from the Combined Pharmacotherapies and Behavioral Interventions for Alcohol Dependence (COMBINE) study. Arch. Gen. Psychiatry 65, 135–144 (2008).
van der Zwaluw, C. S. et al. Polymorphisms in the μ-opioid receptor gene (OPRM1) and the implications for alcohol dependence in humans. Pharmacogenomics 8, 1427–1436 (2007).
Hernandez-Avila, C. A., Wand, G., Luo, X., Gelernter, J. & Kranzler, H. R. Association between the cortisol response to opioid blockade and the Asn40Asp polymorphism at the mu-opioid receptor locus (OPRM1). Am. J. Med. Gen. B Neuropsych. Genet. 118B, 60–65 (2003).
Blomeyer, D. et al. Interaction between CRHR1 gene and stressful life events predicts adolescent heavy alcohol use. Biol. Psychiatry 63, 146–151 (2008).
Hansson, A. C. et al. Variation at the rat Crhr1 locus and sensitivity to relapse into alcohol seeking induced by environmental stress. Proc. Natl Acad. Sci. USA 103, 15236–15241 (2006).
Sommer, W. H. et al. Upregulation of voluntary alcohol intake, behavioral sensitivity to stress, and amygdala crhr1 expression following a history of dependence. Biol. Psychiatry 63, 139–145 (2008).
Koob, G. F., Everitt, B. J. & Robbins, T. W. in Fundamental Neuroscience 3rd edn (eds Squire, L. G. et al.). 987–1016 (Academic Press, Amsterdam, 2008).
Collins, R. J., Weeks, J. R., Cooper, M. M., Good, P. I. & Russell, R. R. Prediction of abuse liability of drugs using IV self-administration by rats. Psychopharmacology 82, 6–13 (1984).
Kornetsky, C. & Bain, G. in Modern Methods in Pharmacology: Testing and Evaluation of Drugs of Abuse Vol. 6 (eds Adler, M. W. & Cowan, A.) 211–231 (Wiley-Liss, New York, 1990).
Tornatzky, W. & Miczek, K. A. Cocaine self-administration “binges”: transition from behavioral and autonomic regulation toward homeostatic dysregulation in rats. Psychopharmacology 148, 289–298 (2000).
Ahmed, S. H. & Koob, G. F. Transition from moderate to excessive drug intake: change in hedonic set point. Science 282, 298–300 (1998).
Kelly, T. H., Foltin, R. W., Emurian, C. S. & Fischman, M. W. Are choice and self-administration of marijuana related to Δ9-THC content? Exp. Clin. Psychopharmacol. 5, 74–82 (1997).
Haney, M., Foltin, R. W. & Fischman, M. W. Effects of pergolide on intravenous cocaine self-administration in men and women. Psychopharmacology 137, 15–24 (1998).
de Wit, H. Impulsivity as a determinant and consequence of drug use: a review of underlying processes. Addict. Biol. 14, 22–31 (2009).
Rachlin, H. & Green, L. Commitment, choice and self-control. J. Exp. Anal. Behav. 17, 15–22 (1972).
Leth-Steensen, C., Elbaz, Z. K. & Douglas, V. I. Mean response times, variability, and skew in the responding of ADHD children: a response time distributional approach. Acta Psychol. 104, 167–190 (2000).
Schulteis, G., Yackey, M., Risbrough, V. & Koob, G. F. Anxiogenic-like effects of spontaneous and naloxone-precipitated opiate withdrawal in the elevated plus-maze. Pharmacol. Biochem. Behav. 60, 727–731 (1998).
Tzschentke, T. M. Measuring reward with the conditioned place preference paradigm: a comprehensive review of drug effects, recent progress and new issues. Prog. Neurobiol. 56, 613–672 (1998).
Markou, A., Kosten, T. R. & Koob, G. F. Neurobiological similarities in depression and drug dependence: a self-medication hypothesis. Neuropsychopharmacology 18, 135–174 (1998).
Ahmed, S. H., Walker, J. R. & Koob, G. F. Persistent increase in the motivation to take heroin in rats with a history of drug escalation. Neuropsychopharmacology 22, 413–421 (2000).
Roberts, A. J., Heyser, C. J., Cole, M., Griffin, P. & Koob, G. F. Excessive ethanol drinking following a history of dependence: animal model of allostasis. Neuropsychopharmacology 22, 581–594 (2000).
Kitamura, O., Wee, S., Specio, S. E., Koob, G. F. & Pulvirenti, L. Escalation of methamphetamine self-administration in rats: a dose-effect function. Psychopharmacology 186, 48–53 (2006).
O'Dell, L. E. & Koob, G. F. “Nicotine deprivation effect” in rats with intermittent 23-hour access to intravenous nicotine self-administration. Pharmacol. Biochem. Behav. 86, 346–353 (2007).
Hjalmarson, A. I. Effect of nicotine chewing gum in smoking cessation: a randomized, placebo-controlled, double-blind study. J. Am. Med. Assoc. 252, 2835–2838 (1984).
Haney, M. The marijuana withdrawal syndrome: diagnosis and treatment. Curr. Psychiatry Rep. 7, 360–366 (2005).
Rusted, J. M., Caulfield, D., King, L. & Goode, A. Moving out of the laboratory: does nicotine improve everyday attention? Behav. Pharmacol. 11, 621–629 (2000).
Lawrence, N. S., Ross, T. J. & Stein, E. A. Cognitive mechanisms of nicotine on visual attention. Neuron 36, 539–548 (2002).
Giessing, C., Thiel, C. M., Rösler, F. & Fink, G. R. The modulatory effects of nicotine on parietal cortex activity in a cued target detection task depend on cue reliability. Neuroscience 137, 853–864 (2006).
Christensen, J. K., Moller, I. W., Ronsted, P., Angelo, H. R. & Johansson, B. Dose-effect relationship of disulfiram in human volunteers. I: Clinical studies. Pharmacol. Toxicol. 68, 163–165 (1991).
Goedde, H. W., Agarwal, D. P. & Harada, S. in Isozymes: Current Topics in Biological and Medical Research: Cellular Localization, Metabolism, and Physiology Vol. 8 (eds Rattazzi, M. C., Scandalios, J. G. & Whitt, G. S.) 175–193 (Liss, New York, 1983).
Volpicelli, J. R., Alterman, A. I., Hayashida, M. & O'Brien, C. P. Naltrexone in the treatment of alcohol dependence. Arch. Gen. Psychiatry 49, 876–880 (1992).
O'Malley, S. S. et al. Naltrexone and coping skills therapy for alcohol dependence: a controlled study. Arch. Gen. Psychiatry 49, 881–887 (1992).
Hurt, R. D. et al. A comparison of sustained-release bupropion and placebo for smoking cessation. N. Engl. J. Med. 337, 1195–1202 (1997).
Mason, B. J. Acamprosate and naltrexone treatment for alcohol dependence: an evidence-based risk-benefits assessment. Eur. Neuropsychopharmacol. 13, 469–475 (2003). This paper provides an evidence-based risk–benefit assessment of acamprosate and naltrexone in the treatment of alcohol dependence. The safety of the two drugs in combination is supported by two independent double-blind studies, suggesting that combination treatment is a realistic goal.
Mason, B. J., Goodman, A. M., Chabac, S. & Lehert, P. Effect of oral acamprosate on abstinence in patients with alcohol dependence in a double-blind, placebo-controlled trial: the role of patient motivation. J. Psychiatr. Res. 40, 383–393 (2006).
Jorenby, D. E. et al. Efficacy of varenicline, an α4β2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. J. Am. Med. Assoc. 296, 56–63 (2006); erratum 296, 1355 (2006).
Altshuler, H. L., Phillips, P. E. & Feinhandler, D. A. Alteration of ethanol self-administration by naltrexone. Life Sci. 26, 679–688 (1980).
Heyser, C. J., Moc, K. & Koob, G. F. Effects of naltrexone alone and in combination with acamprosate on the alcohol deprivation effect in rats. Neuropsychopharmacology 28, 1463–1471 (2003).
Colombo, G. et al. Ability of baclofen in reducing alcohol intake and withdrawal severity: I. Preclinical evidence. Alcohol. Clin. Exp. Res. 24, 58–66 (2000).
Backstrom, P. & Hyytia, P. Suppression of alcohol self-administration and cue-induced reinstatement of alcohol seeking by the mGlu2/3 receptor agonist LY379268 and the mGlu8 receptor agonist (S)-3, 4-DCPG. Eur. J. Pharmacol. 528, 110–118 (2005).
Le Magnen, J., Tran, G., Durlach, J. & Martin, C. Dose-dependent suppression of the high alcohol intake of chronically intoxicated rats by Ca-acetyl homotaurinate. Alcohol 4, 97–102 (1987).
Liu, X. & Weiss, F. Additive effect of stress and drug cues on reinstatement of ethanol seeking: exacerbation by history of dependence and role of concurrent activation of corticotropin-releasing factor and opioid mechanisms. J. Neurosci. 22, 7856–7861 (2002).
Bachteler, D., Economidou, D., Danysz, W., Ciccocioppo, R. & Spanagel, R. The effects of acamprosate and neramexane on cue-induced reinstatement of ethanol-seeking behavior in rat. Neuropsychopharmacology 30, 1104–1110 (2005).
Maccioni, P., Bienkowski, P., Carai, M. A., Gessa, G. L. & Colombo, G. Baclofen attenuates cue-induced reinstatement of alcohol-seeking behavior in Sardinian alcohol-preferring (sP) rats. Drug Alcohol Depend. 95, 284–287 (2008).
Le, A. D., Harding, S., Juzytsch, W., Funk, D. & Shaham, Y. Role of alpha-2 adrenoceptors in stress-induced reinstatement of alcohol seeking and alcohol self-administration in rats. Psychopharmacology 179, 366–373 (2005).
Acknowledgements
This is manuscript number 19,996 authored from The Scripps Research Institute. The authors thank M. Arends for his assistance with manuscript preparation. Preparation of this work was supported by the Pearson Center for Alcoholism and Addiction Research and National Institutes of Health grants AA12602, AA08459, (R37)AA014028 and AA06420 from the National Institute on Alcohol Abuse and Alcoholism, DA04043, DA04398, (P20)DA024194 and DA10072 from the National Institute on Drug Abuse, DK26741 from the National Institute of Diabetes and Digestive and Kidney Diseases, and 17RT-0095 from the Tobacco-Related Disease Research Program from the State of California. The opinions expressed in this article by G.K.L. are as an individual and not as an employee of Nereus Pharmaceuticals.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
George Koob acts as a consultant for Alkermes, Boehringer-Ingelheim, GlaxoSmithKline, Eli Lilly, Abbott, Addex, Psychogenics and Transept Pharmaceuticals.Barbara Mason acts as a consultant for Catalyst Pharmaceutical Partners, Forest Laboratories, Jonson & Johnson and GlaxoSmithKline; is on the Scientific Advisory Board of Addex Pharmaceuticals; ison the Speakers Bureau of PremPharm.
Supplementary information
Supplementary information S2 (box)
Investigational new drug application (PDF 198 kb)
Related links
Related links
FURTHER INFORMATION
Small Business Innovation Research (SBIR) and Small Business Technology Transfer (STTR) programmes
Glossary
- Addiction
-
This term can be used interchangeably with substance dependence (as currently defined by the Diagnostic and Statistical Manual of Mental Disorders, 4th edition) to refer to a final stage of a usage process. Clinically, the occasional but limited use of a drug with the potential for abuse or dependence is distinct from the emergence of addiction.
- Face-valid model
-
A model that looks or seems to be a valid representation of what it purports to measure.
- Kleptomania
-
A classic impulse control disorder in which there is an increase in tension before stealing an object or objects that are not needed and relief after the act, but little or no regret or self-reproach.
- Obsessive–compulsive disorder
-
A classic compulsive disorder is one in which obsessions of contamination or harm drive anxiety, the reduction of which requires repetitive compulsive acts to reduce the anxiety.
- Binge
-
Any behaviour indulged to excess. In alcohol abuse, a binge is defined in the United States as four drinks for females and five drinks for males in a 2-hour period or reaching a blood alcohol level of 0.08 g per 100 ml.
- Withdrawal
-
A collection of physiological signs and symptoms that present after the sudden cessation of drug intake, which can include shaking, sweating and anxiety, depending on the drug.
- Place conditioning
-
A procedure for assessing the reinforcing efficacy of drugs using a classical or Pavlovian conditioning procedure. Animals typically show conditioned place preference for an environment associated with the common drugs of addiction in humans and avoid environments associated with aversive states of drug withdrawal (that is, they show conditioned place aversion).
- Corticotropin-releasing factor
-
A 41-amino-acid polypeptide with wide distribution throughout the brain and high concentrations in cell bodies in the paraventricular nucleus of the hypothalamus, the basal forebrain and notably the extended amygdala and brainstem.
- GABAA receptor
-
A receptor that is coupled to Cl− channels and forms a receptor complex that includes recognition sites for convulsants, benzodiazepines, barbiturates and steroids.
- GABAB receptor
-
A metabotropic receptor that regulates K+ and Ca2+ channels through a G protein-coupled mechanism. Both GABAA and GABAB receptors have an inhibitory action in the central nervous system and are thought to mediate the anxiety-decreasing, motor-uncoordinating, sedative and hypnotic effects of alcohol.
- Hamilton Depression Inventory
-
A validated scale to measure the severity of depressive symptoms.
- Dynorphins
-
Opioid peptides derived from the prodynorphin precursor that contain the leucine–encephalin sequence at their amino termini. They are the presumed endogenous ligands of the κ-opioid receptor and have long been thought to mediate negative emotional states.
- Behavioural sensitization
-
An increased drug-induced locomotor response or drug reward response with repeated administration.
- Endophenotype
-
Measurable components, unseen by the unaided eye, along the pathway between disease and genotype.
- Multiple event monitoring system cap
-
Caps on pill bottles with built-in microelectronics that record each date and time the cap is removed.
Rights and permissions
About this article
Cite this article
Koob, G., Kenneth Lloyd, G. & Mason, B. Development of pharmacotherapies for drug addiction: a Rosetta Stone approach. Nat Rev Drug Discov 8, 500–515 (2009). https://doi.org/10.1038/nrd2828
Issue Date:
DOI: https://doi.org/10.1038/nrd2828
This article is cited by
-
NGF, BDNF and Arc mRNA Expression in the Hippocampus of Rats After Administration of Morphine
Neurochemical Research (2019)
-
Differences in the subjective and motivational properties of alcohol across alcohol use severity: application of a novel translational human laboratory paradigm
Neuropsychopharmacology (2018)
-
From gene networks to drugs: systems pharmacology approaches for AUD
Psychopharmacology (2018)
-
A genetic reduction in the serotonin transporter differentially influences MDMA and heroin induced behaviours
Psychopharmacology (2018)
-
Effects of the nicotinic agonist varenicline, nicotinic antagonist r-bPiDI, and DAT inhibitor (R)-modafinil on co-use of ethanol and nicotine in female P rats
Psychopharmacology (2018)