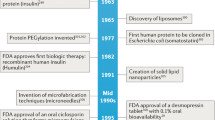
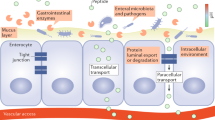
Key Points
-
Macromolecules comprise a growing group of new pharmaceutical products with the potential to treat diseases that heretofore have had no effective therapeutic options.
-
To date, the therapeutic application of these drugs has been limited because they are effective only when administered parenterally.
-
Since the discovery of insulin and heparin in the early 1900s, there has been keen interest in solving the problem of orally delivering macromolecules safely and effectively. Various approaches have been investigated including altering the intestinal membrane, targeting intestinal transport mechanisms, chemically altering the drug and carrier-assisted transport mechanisms.
-
To date, the most promising approach has been the carrier-assisted approach, which has advanced seven different macromolecules or highly charged small molecules human proof-of-concept testing.
Abstract
The rapid integration of new technologies by the pharmaceutical industry has resulted in numerous breakthroughs in the discovery, development and manufacturing of pharmaceutical products. In particular, the commercial-scale production of high-purity recombinant proteins has resulted in important additions to treatment options for many large therapeutic areas. In addition to proteins, other macromolecules, such as the animal-derived mucopolysacharide heparins, have also seen dramatic growth as injectable pharmaceutical products. To date, macromolecules have been limited as therapeutics by the fact that they cannot be orally delivered. This article will address the current status and future possibilities of oral macromolecular drug delivery.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Brange, J. & Langkjaer, L. in Protein Delivery: Physical Systems (eds Sanders, L. M. & Hendren, R. W.) 343–412 (Plenum, New York, 1997).
Chetty, D. J. & Chien, Y. W. Novel methods of insulin delivery: an update. Crit. Rev. Ther. Drug Carrier Syst. 15, 629–670 (1998).
Lever, R. & Page, C. P. Novel drug development opportunities for heparin. Nature Rev. Drug Discov. 1, 140–148 (2002).
Shah, R. B., Ahsan, F. & Khan, M. A. Oral delivery of proteins: progress and prognostication. Crit. Rev. Ther. Drug Carrier Syst. 19, 135–169 (2002).
Lee, H. J. Protein drug oral delivery: the recent progress. Arch. Pharm. Res. 25, 572–584 (2002).
Sood, A. & Panchagnula, R. Peroral route: an opportunity for protein and peptide drug delivery. Chem. Rev. 101, 3275–3303 (2001). Comprehensive review of approaches for oral delivery of macromolecules
Gomez-Orellana, I. & Paton, D. R. Advances in the oral delivery of proteins. Exp. Opin. Ther. Patents 8, 223–234 (1998).
Gomez-Orellana, I. & Paton, D. R. Advances in the oral delivery of proteins. Update. Exp. Opin. Ther. Patents 9, 247–253 (1999).
Donovan, M. D., Flynn, G. L. & Amidon, G. L. Absorption of polyethylene glycols 600 through 2000: the molecular weight dependence of gastrointestinal and nasal absorption. Pharm. Res. 7, 863–868 (1990).
Camenisch, G., Alsenz, J., van de Waterbeemd, H. & Folkers, G. Estimation of permeability by passive diffusion through Caco-2 cell monolayers using the drugs' lipophilicity and molecular weight. Eur. J. Pharm. Sci. 6, 317–324 (1998).
Woodley, J. F. Enzymatic barriers for GI peptide and protein delivery. Crit. Rev. Ther. Drug Carrier Syst. 11, 61–95 (1994).
Rubinstein, A. Approaches and opportunities in colon-specific drug delivery. Crit. Rev. Ther. Drug Carrier Syst. 12, 101–149 (1995).
van den Mooter, G. & Kinget, R. Oral colon-specific drug delivery: a review. Drug Deliv 2, 81–93 (1995).
Tozaki, H. et al. Degradation of insulin and calcitonin and their protection by various protease inhibitors in rat caecal contents: implications in peptide delivery to the colon. J. Pharm. Pharmacol. 49, 164–168 (1997).
Bernkop-Schnurch, A. The use of inhibitory agents to overcome the enzymatic barrier to perorally administered therapeutic peptides and proteins. J. Control. Release 52, 1–16 (1998). Informative review of different approaches developed to protect protein and peptide drugs from proteolytic degradation in the gastrointestinal tract.
Bernkop-Schnurch, A. & Walker, G. Multifunctional matrices for oral peptide delivery. Crit. Rev. Ther. Drug Carrier Syst. 18, 459–501 (2001).
Mathiowitz, E. et al. Biologically erodable microspheres as potential oral drug delivery systems Nature 386, 410–414 (1997).
Jung, T. et al. Biodegradable nanoparticles for oral delivery of peptides: is there a role for polymers to affect mucosal uptake?. Eur. J. Pharm. Biopharm. 50, 147–160 (2000). Recent review of polymeric materials used for oral delivery of macromolecules
Florence, A. T. & Hussain, N. Transcytosis of nanoparticle and dentrimer delivery systems: evolving vistas. Adv. Drug Deliv. Rev. 50, S69–S89 (2001).
Kompella, U. B. & Lee, V. H. L. Delivery systems for penetration enhancement of peptide and protein drugs: design considerations. Adv. Drug Deliv. Rev. 46, 211–245 (2001).
Winiwarter, S. et al. Correlation of human jejunal permeability (in vivo) of drugs with experimentally and theoretically derived parameters. A multivariate data analysis approach. J. Med. Chem. 41, 4939–4949 (1998).
Lennernas, H. Human intestinal permeability. J. Pharm. Sci. 87, 403–410 (1998).
Daugherty, A. L. & Mrsny, R. J. Regulation of the intestinal epithelial paracellular barrier. Pharm. Sci. Technol. Today 2, 281–287 (1999). Comprehensive review of paracellular absorption mechanims.
Madara, J. L. Modulation of tight junctional permeability. Adv. Drug Deliv. Rev. 41, 251–253 (2000).
Swenson, E. S. & Curatolo, W. J. Intestinal permeability enhancement for proteins, peptides and other polar drugs: mechanisms and potential toxicity. Adv. Drug Deliv. Rev. 8, 39–92 (1992).
Daugherty, A. L. & Mrsny, R. J. Transcellular uptake mechanisms of the intestinal epithelial barrier. Part one. Pharm. Sci. Technol. Today 4, 144–151 (1999).
Chao, A. C. et al. Enhancement of intestinal model compound transport by DS-1, a modified Quillaja saponin. J. Pharm. Sci. 87, 1395–1399 (1998).
Fix, J. A. Strategies for delivery of peptides utilizing absorption-enhancing agents. J. Pharm. Sci. 85, 1282–1285 (1996).
Dorkoosh, F. A. et al. Effects of superporous hydrogels on paracellular drug permeability and cytotoxicity studies in Caco-2 cell monolayers. Int. J. Pharm. 241, 35–45 (2002).
Thanou, M., Verhoef, J. C., Junginger, H. E. Oral drug absorption enhancement by chitosan and its derivatives. Adv. Drug Deliv. Rev. 52, 117–126 (2001).
Torres-Lugo, M., Garcia, M., Record, R. & Peppas, N. A. pH-Sensitive hydrogels as gastrointestinal tract absorption enhancers: transport mechanisms of salmon calcitonin and other model molecules using the Caco-2 cell model. Biotechnol. Prog. 18, 612–616 (2002).
Ward, P. D., Tippin, T. K. & Thakker, D. R. Enhancing paracellular permeability by modulating epithelial tight junctions. Pharm. Sci. Technol. Today 3, 346–358 (2000).
Fasano, A. & Uzzau, S. Modulation of intestinal tight junctions by Zonula occludens toxin permits enteral administration of insulin and other macromolecules in an animal model. J. Clin. Invest. 99, 1158–1164 (1997).
Yamamoto, A. et al. Modulation of intestinal permeability by nitric oxide donors: implications in intestinal delivery of poorly absorbable drugs. J. Pharmacol. Exp. Ther. 296, 84–90 (2001).
Numata, N. et al. Improvement of intestinal absorption of macromolecules by nitric oxide donor. J. Pharm. Sci. 89, 1296–1304 (2000).
Watanabe, Y. et al. Absorption enhancement of a protein drug by nitric oxide donor: effect on nasal absorption of human granulocyte colony-stimulating factor. J. Drug Target. 8, 185–194 (2000).
Yen, W. C. & Lee, V. H. Role of Na+ in the asymmetric paracellular transport of 4-phenylazobenzyloxycarbonyl-L-Pro-L-Leu-Gly-L-Pro-D-Arg across rabbit colonic segments and Caco-2 cell monolayers. J. Pharmacol. Exp. Ther. 275, 114–119 (1995).
Raoof, A. A. et al. Effect of sodium caprate on the intestinal absorption of two modified antisense oligonucleotides in pigs. Eur. J. Pharm. Sci. 17, 131–138 (2002).
Hosny, E. A., Elkheshen, S. A. & Saleh, S. I. Buccoadhesive tablets for insulin delivery: in vitro and in vivo studies. Boll. Chim. Farm. 141, 210–217 (2002).
Modi, P., Mihic, M. & Lewin, A. The evolving role of oral insulin in the treatment of diabetes using a novel RapidMist System. Diabetes Metab. Res. Rev. 18, S38–S42 (2002).
Dyer, A. M. et al. Nasal delivery of insulin using novel chitosan based formulations: a comparative study in two animal models between simple chitosan formulations and chitosan nanoparticles. Pharm. Res. 19, 998–1008 (2002).
Callens, C., Pringels, E. & Remon, J. P. Influence of multiple nasal administrations of bioadhesive powders on the insulin bioavailability. Int. J. Pharm. 250, 415–422 (2003).
Sayani, A. P. & Chien, Y. W. Systemic delivery of peptides and proteins across absorptive mucosae. Crit. Rev. Ther. Drug Carrier Syst. 13, 85–184 (1996).
Williams, G. C. & Sinko, P. J. Oral absorption of the HIV protease inhibitors: a current update. Adv. Drug Deliv. Rev. 39, 211–238 (1999).
Porter, C. J. & Charman, W. N. In vitro assessment of oral lipid based formulations. Adv. Drug Deliv. Rev. 50, S127–S147 (2001).
Rubio-Aliaga, I. & Daniel, H. Mammalian peptide transporters as targets for drug delivery. Trends Pharmacol. Sci. 23, 434–440 (2002).
Swaan, P. W. Recent advances in intestinal macromolecular drug delivery via receptor-mediated transport pathways. Pharm. Res. 15, 826–834 (1998). Informative review of receptor-mediated transport systems available in the gastrointestinal tract and their potential for facilitating macromolecule absorption.
Oh, D. M., Han, H. K. & Amidon, G. L. Drug transport and targeting. Intestinal transport. Pharm. Biotechnol. 12, 59–88 (1999).
Rouquayrol, M., Gaucher, B., Roche, D., Greiner, J. & Vierling, P. Transepithelial transport of prodrugs of the HIV protease inhibitors saquinavir, indinavir, and nelfinavir across Caco-2 cell monolayers. Pharm. Res. 19, 1704–1712 (2002).
Nielsen, C. U. et al. Dipeptide model prodrugs for the intestinal oligopeptide transporter. Affinity for and transport via hPepT1 in the human intestinal Caco-2 cell line. J. Control. Release 76, 129–138 (2001).
Borchardt, R., Aube, J., Siahaan, T. J., Gangwar, S. & Pauletti, G. M. Improvement of oral peptide bioavailability: Peptidomimetics and prodrug strategies. Adv. Drug Deliv. Rev. 27, 235–256 (1997).
Kramer, W. et al. Intestinal absorption of bile acids: paradoxical behaviour of the 14 kDa ileal lipid-binding protein in differential photoaffinity labelling. Biochem. J. 333, 335–341 (1998).
Swaan, P. W., Hillgren, K. M., Szoka, F. C. Jr. & Oie, S. Enhanced transepithelial transport of peptides by conjugation to cholic acid. Bioconjug. Chem. 8, 520–525 (1997).
Russell-Jones, G. J., Westwood, S. W. & Habberfield, A. D. Vitamin B12 mediated oral delivery systems for granulocyte-colony stimulating factor and erythropoietin. Bioconjug. Chem. 6, 459–465 (1995).
Russell-Jones, G. J. Use of vitamin B12 conjugates to deliver protein drugs by the oral route. Crit. Rev. Ther. Drug Carrier Syst. 15, 557–586 (1998).
Habberfield, A., Jensen-Pippo, K., Ralph, L., Westwood, S. & Russel-Jones, G. J. Vitamin B12-mediated uptake of recombinant therapeutic proteins from the gut. Int. J. Pharm. 145, 1–8 (1996).
Spiekermann, G. M. et al. Receptor-mediated immunoglobulin G transport across mucosal barriers in adult life: functional expression of FcRn in the mammalian lung. J. Exp. Med. 196, 303–310 (2002).
Neutra, M. R., Wilson, J. M., Weltzin, R. A. & Kraehenbuhl, J. P. Membrane domains and macromolecular transport in intestinal epithelial cells. Am. Rev. Respir. Dis. 138, S10–S16 (1988).
Russell-Jones, G. J., Arthur, L. & Walker, H. Vitamin B12-mediated transport of nanoparticles across Caco-2 cells. Int. J. Pharm. 179, 247–255 (1999).
Clackson, T. & Wells, J. A. A hot spot of binding energy in a hormone-receptor interface. Science 267, 383–386 (1995).
Wrighton, N. C. et al. Small peptides as potent mimetics of the protein hormone erythropoietin. Science 273, 458–463 (1996).
Maslov, D. L., Lokhov, P. G., Abakumova, O. Y., Tsvetkova, T. A. & Prozorovskiy, V. N. J. New peptidomimetics of insulin. J. Biochem. Mol. Biol. Biophys. 6, 261–265 (2002)
Cwirla, S. E. et al. Peptide agonist of the thrombopoietin receptor as potent as the natural cytokine. Science 276, 1696–1699 (1997).
Clement, S., Still, J. G. & Kosutic, G. Oral insulin product hexyl-insulin monoconjugate 2 (HIM2) in type 1 diabetes mellitus: The glucose stabilization effects of HIM2. Diabetes Technol. Ther. 4, 459–466 (2002).
Kipnes, M., Dandona, P., Tripathy, D., Still, J. G. & Kosutic, G. Control of postprandial plasma glucose by an oral insulin product (HIM2) in patients with type 2 diabetes. Diabetes Care 26, 421–426 (2003).
Asada, H. et al. Absorption characteristics of chemically modified-insulin derivatives with various fatty acids in the small and large intestine. J. Pharm. Sci. 84, 682–687 (1995).
Lee, Y., Nam, J. H., Shin, H. C. & Byun, Y. Conjugation of low-molecular-weight heparin and deoxycholic acid for the development of a new oral anticoagulant agent. Circulation 104, 3116–3120 (2001).
Uchiyama, T. et al. Development of novel lipophilic derivatives of DADLE (leucine enkephalin analogue): intestinal permeability characateristics of DADLE derivatives in rats. Pharm. Res. 17, 1461–1467 (2000).
Yodoya, E. et al. Enhanced permeability of tetragastrin across the rat intestinal membrane and its reduced degradation by acylation with various fatty acids. J. Pharmacol. Exp. Ther. 271, 1509–1513 (1994).
Hashizume, M. et al. Improvement of large intestinal absorption of insulin by chemical modification with palmitic acid in rats. J. Pharm. Pharmacol. 44, 555–559 (1992).
Yamada, K., Murakami, M., Yamamoto, A., Takada, K. & Muranishi, S. Improvement of intestinal absorption of thyrotropin-releasing hormone by chemical modification with lauric acid. J. Pharm. Pharmacol. 44, 717–721 (1992).
Masuda, K., Horie, K., Suzuki, R., Yoshikawa, T. & Hirano, K. Oral delivery of antigens in liposomes with some lipid compositions modulates oral tolerance to the antigens. Microbiol. Immunol. 46, 55–58 (2002).
Lasic, D. D. Novel applications of liposomes. Trends. Biotechnol. 16, 307–321 (1998).
Porter, C. J. Drug delivery to the lymphatic system. Crit. Rev. Ther. Drug Carrier Syst. 14, 333–393 (1997).
Abbas, R. et al. Oral insulin: pharmacokinetics and pharmacodynamics of human insulin following oral administration of an insulin/delivery agent capsule in healthy volunteers. Diabetes 51, A48 (2002).
Baughman, R. A. et al. Oral delivery of anticoagulant doses of heparin. A randomized, double-blind, controlled study in humans. Circulation 98, 1610–1615 (1998).
Buclin, T., Rochat, M. C., Burckhardt, P., Azria, M. & Attinger, M. Bioavailability and biological efficacy of new oral formulation of salmon calcitonin in healthy volunteers. J. Bone Miner. Res. 17, 1478–1485 (2002).
Milstein, S. J. et al. Partially unfolded proteins efficiently penetrate cell membranes- implications for oral drug delivery. J. Control. Release 53, 259–267 (1998).
Engelman, D. M. & Steitz, T. A. The spontaneous insertion of proteins into and across membranes: the helical hairpin hypothesis. Cell 23, 411–422 (1981).
Sabatini, D. D., Kreibich, G., Morimoto, T. & Adesnik, M. Mechanisms for the incorporation of proteins in membranes and organelles. J. Cell Biol. 92, 1–22 (1982).
Zimmerman, R. & Meyer, D. I. A year of new insights into how proteins cross membranes. Trends Biochem. Sci. 11, 512–515 (1986).
van der Goot, F. G., Gonzalez-Manas, J. M., Lakey, J. H. & Pattus, F. A 'molten-globule' membrane-insertion intermediate of the pore-forming domain of colicin A. Nature 354, 408–10 (1991).
Sanz, J. M. & Gimenez-Gallego, G. A partly folded state of acidic fibroblast growth factor at low pH. Eur. J. Biochem. 246, 328–35 (1997).
Leone-Bay, A. et al. Oral delivery of biologically active parathyroid hormone. Pharm. Res. 18, 964–970 (2001).
Brayden, D. et al. Heparin absorption across the intestine: Effects of sodium N-[8(2-hydroxybenzoyl) amino]caprylate in rat in situ intestinal instillations and in CACO-2 monolayers. Pharm. Res. 14, 1772–1779 (1997).
Wu, S. -J. & Robinson, J. R. Transcellular and lipophilic complex-enhanced intestinal absorption of human growth hormone. Pharm. Res. 16, 1266–1272 (1999).
Malkov, D., Wang, H., Dinh, S. & Gomez-Orellana, I. Pathway of oral absorption of heparin with sodium N-[8-(2-hydroxylbenzoyl)amino]caprylate. Pharm. Res. 19, 1180–1184 (2002).
Stoll, B. R., Leipold, H., Milstein, S. & Edwards, D. A. A mechanistic analysis of carrier–mediated oral delivery of protein therapeutics. J. Control. Release 64, 217–228 (2000).
Author information
Authors and Affiliations
Corresponding authors
Related links
Related links
DATABASES
LocusLink
FURTHER INFORMATION
Encyclopedia of Life Sciences
Glossary
- BIOAVAILABILITY
-
The extent to which a drug becomes available in the bloodstream, or at the target tissue, after administration.
- LIPOPHILICITY
-
The extent of insolubility in water or resistance to hydration of a molecule or chemical entity.
- HYDROPHILICITY
-
The extent of solubility in water or the ability to be hydrated of a molecule or chemical entity.
- TRANSCYTOSIS
-
The process by which polarized cells transport certain molecules by engulfing them into vesicles that are released at the opposite side of the cells.
- ENTEROCYTES
-
Epithelial cells that form the gastrointestinal wall.
- TIGHT JUNCTION
-
An intercellular junction that seals together adjacent epithelial cells, preventing the passage of substances through the spaces in between cells.
- FUNCTIONAL GROUP
-
A chemical group with specific properties attached to a molecule.
- PHARMACOKINETICS
-
The way in which drugs are absorbed, distributed, localized in tissues, metabolized and excreted over a period of time.
Rights and permissions
About this article
Cite this article
Goldberg, M., Gomez-Orellana, I. Challenges for the oral delivery of macromolecules. Nat Rev Drug Discov 2, 289–295 (2003). https://doi.org/10.1038/nrd1067
Issue Date:
DOI: https://doi.org/10.1038/nrd1067
This article is cited by
-
Cracking the intestinal lymphatic system window utilizing oral delivery vehicles for precise therapy
Journal of Nanobiotechnology (2023)
-
Bacterial therapies at the interface of synthetic biology and nanomedicine
Nature Reviews Bioengineering (2023)
-
Oral delivery of RNAi for cancer therapy
Cancer and Metastasis Reviews (2023)
-
SNAC for Enhanced Oral Bioavailability: An Updated Review
Pharmaceutical Research (2023)
-
Multi-functional self-assembly nanoparticles originating from small molecule natural product for oral insulin delivery through modulating tight junctions
Journal of Nanobiotechnology (2022)