Key Points
-
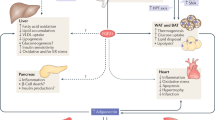
Endocrine fibroblast growth factors (FGFs) are critical regulators of glucose and lipid metabolism, and gatekeepers of vitamin D and phosphate homeostasis.
-
Endocrine FGFs may serve as both risk factors and biomarkers of chronic metabolic disorders, including obesity, type 2 diabetes, cancer, and kidney and cardiovascular disease.
-
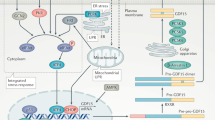
The intestine-derived FGF19 is a critical regulator of both bile acid and glucose metabolism, and its pharmacological modulation has been proposed as a promising approach for the treatment of disorders related to the gut–liver axis, including cholestasis.
-
The liver-derived hormone FGF21 is a master regulator of substrate turnover and orchestrates crosstalk between the liver, white adipose tissue, brain, pancreas and brown adipose tissue. FGF21 is rapidly emerging as an attractive target in the treatment of metabolic syndrome and type 2 diabetes.
-
The bone-derived hormone FGF23 is the physiological regulator of phosphate and vitamin D serum levels and, along with its cofactor α-klotho, it could serve as an effective biomarker for prediction of outcome as well as treatment guidance in chronic kidney disease. A health-promoting activity of FGF23 has also been proposed.
-
Pharmaceutical efforts are in progress to develop FGF analogues or mimetics that are devoid of mitogenic potential and risk of bone loss, and also endowed with a better pharmacokinetic profile.
Abstract
The endocrine fibroblast growth factors (FGFs), FGF19, FGF21 and FGF23, are critical for maintaining whole-body homeostasis, with roles in bile acid, glucose and lipid metabolism, modulation of vitamin D and phosphate homeostasis and metabolic adaptation during fasting. Given these functions, the endocrine FGFs have therapeutic potential in a wide array of chronic human diseases, including obesity, type 2 diabetes, cancer, and kidney and cardiovascular disease. However, the safety and feasibility of chronic endocrine FGF administration has been challenged, and FGF analogues and mimetics are now being investigated. Here, we discuss current knowledge of the complex biology of the endocrine FGFs and assess how this may be harnessed therapeutically.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Beenken, A. & Mohammadi, M. The FGF family: biology, pathophysiology and therapy. Nat. Rev. Drug Discov. 8, 235–253 (2008).
Itoh, N. & Ornitz, D. M. Evolution of the Fgf and Fgfr gene families. Trends Genet. 20, 563–569 (2004).
Itoh, N. & Ornitz, D. M. Functional evolutionary history of the mouse Fgf gene family. Dev. Dyn. 237, 18–27 (2008).
Ornitz, D. M. et al. Receptor specificity of the fibroblast growth factor family. J. Biol. Chem. 271, 15292–15297 (1996).
Ornitz, D. M. FGFs, heparan sulfate and FGFRs: complex interactions essential for development. Bioessays 22, 108–112 (2000).
Mohammadi, M., Olsen, S. K. & Ibrahimi, O. A. Structural basis for fibroblast growth factor receptor activation. Cytokine Growth Factor Rev. 16, 107–137 (2005).
Itoh, N. Hormone-like (endocrine) Fgfs: their evolutionary history and roles in development, metabolism, and disease. Cell Tissue Res. 342, 1–11 (2010).
Goetz, R. et al. Molecular insights into the klotho-dependent, endocrine mode of action of fibroblast growth factor 19 subfamily members. Mol. Cell. Biol. 27, 3417–3428 (2007).
Zhang, X. et al. Receptor specificity of the fibroblast growth factor family. The complete mammalian FGF family. J. Biol. Chem. 281, 15694–15700 (2006).
Kurosu, H. et al. Regulation of fibroblast growth factor-23 signaling by klotho. J. Biol. Chem. 281, 6120–6123 (2006). This paper provides the first evidence that FGF23 requires α-klotho to activate FGFR.
Ogawa, Y. et al. βKlotho is required for metabolic activity of fibroblast growth factor 21. Proc. Natl Acad. Sci. USA 104, 7432–7437 (2007).
Urakawa, I. et al. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature 444, 770–774 (2006). This work identifies the requirement for klotho to convert canonical FGF into endocrine FGFs.
Wu, X. et al. Co-receptor requirements for fibroblast growth factor-19 signaling. J. Biol. Chem. 282, 29069–29072 (2007).
Ding, X. et al. βKlotho is required for fibroblast growth factor 21 effects on growth and metabolism. Cell. Metab. 16, 387–393 (2012).
Kharitonenkov, A. et al. FGF-21/FGF-21 receptor interaction and activation is determined by βKlotho. J. Cell. Physiol. 215, 1–7 (2008).
Lin, B. C., Wang, M., Blackmore, C. & Desnoyers, L. R. Liver-specific activities of FGF19 require Klotho beta. J. Biol. Chem. 282, 27277–27284 (2007).
Kurosu, H. et al. Tissue-specific expression of βKlotho and fibroblast growth factor (FGF) receptor isoforms determines metabolic activity of FGF19 and FGF21. J. Biol. Chem. 282, 26687–26695 (2007). This work establishes β-klotho tissue-specific expression as a major determinant of FGF19 and FGF21 metabolic activity.
Adams, A. C., Cheng, C. C., Coskun, T. & Kharitonenkov, A. FGF21 requires βklotho to act in vivo. PLoS ONE 7, e49977 (2012).
Yang, C. et al. Differential specificity of endocrine FGF19 and FGF21 to FGFR1 and FGFR4 in complex with KLB. PLoS ONE 7, e33870 (2012).
Inagaki, T. et al. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell. Metab. 2, 217–225 (2005). This is the first elucidation of FGF15/19 in bile acid metabolism.
Choi, M. et al. Identification of a hormonal basis for gallbladder filling. Nat. Med. 12, 1253–1255 (2006).
Holt, J. A. et al. Definition of a novel growth factor-dependent signal cascade for the suppression of bile acid biosynthesis. Genes Dev. 17, 1581–1591 (2003).
Song, K. H., Li, T., Owsley, E., Strom, S. & Chiang, J. Y. Bile acids activate fibroblast growth factor 19 signaling in human hepatocytes to inhibit cholesterol 7α-hydroxylase gene expression. Hepatology 49, 297–305 (2009).
Potthoff, M. J. et al. FGF15/19 regulates hepatic glucose metabolism by inhibiting the CREB-PGC-1α pathway. Cell. Metab. 13, 729–738 (2011).
Kir, S. et al. FGF19 as a postprandial, insulin-independent activator of hepatic protein and glycogen synthesis. Science 331, 1621–1624 (2011).
Xu, J. et al. Acute glucose-lowering and insulin-sensitizing action of FGF21 in insulin-resistant mouse models — association with liver and adipose tissue effects. Am. J. Physiol. Endocrinol. Metab. 297, E1105–E1114 (2009).
Kharitonenkov, A. et al. FGF-21 as a novel metabolic regulator. J. Clin. Invest. 115, 1627–1635 (2005).
Coskun, T. et al. Fibroblast growth factor 21 corrects obesity in mice. Endocrinology 149, 6018–6027 (2008).
Bhatnagar, S., Damron, H. A. & Hillgartner, F. B. Fibroblast growth factor-19, a novel factor that inhibits hepatic fatty acid synthesis. J. Biol. Chem. 284, 10023–10033 (2009).
Xu, J. et al. Fibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in diet-induced obese mice. Diabetes 58, 250–259 (2009).
Shimada, T. et al. Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J. Clin. Invest. 113, 561–568 (2004).
Potthoff, M. J. et al. FGF21 induces PGC-1α and regulates carbohydrate and fatty acid metabolism during the adaptive starvation response. Proc. Natl Acad. Sci. USA 106, 10853–10858 (2009). This work elucidates the role of FGF21 in the regulation of carbohydrate and fatty acid metabolism.
Inagaki, T. et al. Endocrine regulation of the fasting response by PPARα-mediated induction of fibroblast growth factor 21. Cell. Metab. 5, 415–425 (2007).
Badman, M. K. et al. Hepatic fibroblast growth factor 21 is regulated by PPARα and is a key mediator of hepatic lipid metabolism in ketotic states. Cell. Metab. 5, 426–437 (2007). This work establishes FGF21 as a crucial hormone in the body's adaptation to fasting.
White, K. E. et al. Autosomal-dominant hypophosphatemic rickets (ADHR) mutations stabilize FGF-23. Kidney Int. 60, 2079–2086 (2001).
Shimada, T. et al. Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia. Proc. Natl Acad. Sci. USA 98, 6500–6505 (2001).
Benet- Pagès, A., Orlik, P., Strom, T. M. & Lorenz-Depiereux, B. An FGF23 missense mutation causes familial tumoral calcinosis with hyperphosphatemia. Hum. Mol. Genet. 14, 385–390 (2005).
Modica, S. et al. Selective activation of nuclear bile acid receptor FXR in the intestine protects mice against cholestasis. Gastroenterology 142, 355–365 (2012). This study provides the first evidence that FGF19 administration protects mice from cholestasis.
Luo, J. et al. A nontumorigenic variant of FGF19 treats cholestatic liver diseases. Sci. Transl. Med. 6, 247ra100 (2014). This is the first evidence that an FGF19 analogue lowers bile acid synthesis in humans.
Degirolamo, C. et al. Prevention of spontaneous hepatocarcinogenesis in farnesoid X receptor-null mice by intestinal-specific farnesoid X receptor reactivation. Hepatology 61, 161–170 (2015).
Gaich, G. et al. The effects of LY2405319, an FGF21 analog, in obese human subjects with type 2 diabetes. Cell. Metab. 18, 333–340 (2013). This is the first study reporting the effects of an FGF21 analogue in humans.
McWhirter, J. R., Goulding, M., Weiner, J. A., Chun, J. & Murre, C. A novel fibroblast growth factor gene expressed in the developing nervous system is a downstream target of the chimeric homeodomain oncoprotein E2A–Pbx1. Development 124, 3221–3232 (1997).
Nishimura, T., Utsunomiya, Y., Hoshikawa, M., Ohuchi, H. & Itoh, N. Structure and expression of a novel human FGF, FGF-19, expressed in the fetal brain. Biochim. Biophys. Acta 1444, 148–151 (1999).
Katoh, M. & Katoh, M. Evolutionary conservation of CCND1-ORAOV1-FGF19-FGF4 locus from zebrafish to human. Int. J. Mol. Med. 12, 45–50 (2003).
Yamashita, T., Yoshioka, M. & Itoh, N. Identification of a novel fibroblast growth factor, FGF-23, preferentially expressed in the ventrolateral thalamic nucleus of the brain. Biochem. Biophys. Res. Commun. 277, 494–498 (2000).
Nishimura, T., Nakatake, Y., Konishi, M. & Itoh, N. Identification of a novel FGF, FGF-21, preferentially expressed in the liver. Biochim. Biophys. Acta 1492, 203–206 (2000).
Tacer, K. F. et al. Research resource: comprehensive expression atlas of the fibroblast growth factor system in adult mouse. Mol. Endocrinol. 24, 2050–2064 (2010).
Wright, T. J. et al. Mouse FGF15 is the ortholog of human and chick FGF19, but is not uniquely required for otic induction. Dev. Biol. 269, 264–275 (2004).
Xie, M. H. et al. FGF-19, a novel fibroblast growth factor with unique specificity for FGFR4. Cytokine 11, 729–735 (1999).
Wente, W. et al. Fibroblast growth factor-21 improves pancreatic β-cell function and survival by activation of extracellular signal-regulated kinase 1/2 and Akt signaling pathways. Diabetes 55, 2470–2478 (2006).
Zhang, X. et al. Serum FGF21 levels are increased in obesity and are independently associated with the metabolic syndrome in humans. Diabetes 57, 1246–1253 (2008).
Muise, E. S. et al. Adipose fibroblast growth factor 21 is up-regulated by peroxisome proliferator-activated receptor γ and altered metabolic states. Mol. Pharmacol. 74, 403–412 (2008).
Kharitonenkov, A. & Shanafelt, A. B. FGF21: a novel prospect for the treatment of metabolic diseases. Curr. Opin. Investig. Drugs 10, 359–364 (2009).
Izumiya, Y. et al. FGF21 is an Akt-regulated myokine. FEBS Lett. 582, 3805–3810 (2008).
Riminucci, M. et al. FGF-23 in fibrous dysplasia of bone and its relationship to renal phosphate wasting. J. Clin. Invest. 112, 683–692 (2003).
Liu, S. et al. Regulation of fibroblastic growth factor 23 expression but not degradation by PHEX. J. Biol. Chem. 278, 37419–37426 (2003).
Nagano, M. et al. Regulation of bile acid synthesis under reconstructed enterohepatic circulation in rats. Steroids 69, 701–709 (2004).
Kim, I. et al. Differential regulation of bile acid homeostasis by the farnesoid X receptor in liver and intestine. J. Lipid Res. 48, 2664–2672 (2007).
Miyata, M. et al. Involvement of multiple elements in FXR-mediated transcriptional activation of FGF19. J. Steroid Biochem. Mol. Biol. 132, 41–47 (2012).
Schmidt, D. R. et al. Regulation of bile acid synthesis by fat-soluble vitamins A and D. J. Biol. Chem. 285, 14486–14494 (2010).
Henkel, A. S., Anderson, K. A., Dewey, A. M., Kavesh, M. H. & Green, R. M. A chronic high-cholesterol diet paradoxically suppresses hepatic CYP7A1 expression in FVB/NJ mice. J. Lipid Res. 52, 289–298 (2011).
Miyata, M., Hata, T., Yamazoe, Y. & Yoshinari, K. SREBP-2 negatively regulates FXR-dependent transcription of FGF19 in human intestinal cells. Biochem. Biophys. Res. Commun. 443, 477–482 (2014).
Wistuba, W., Gnewuch, C., Liebisch, G., Schmitz, G. & Langmann, T. Lithocholic acid induction of the FGF19 promoter in intestinal cells is mediated by PXR. World J. Gastroenterol. 13, 4230–4235 (2007).
Shimizu, M., Li, J., Maruyama, R., Inoue, J. & Sato, R. FGF19 (fibroblast growth factor 19) as a novel target gene for activating transcription factor 4 in response to endoplasmic reticulum stress. Biochem. J. 450, 221–229 (2013).
Vergnes, L., Lee, J. M., Chin, R. G., Auwerx, J. & Reue, K. Diet1 functions in the FGF15/19 enterohepatic signaling axis to modulate bile acid and lipid levels. Cell. Metab. 17, 916–928 (2013).
Wu, X. et al. Selective activation of FGFR4 by an FGF19 variant does not improve glucose metabolism in ob/ob mice. Proc. Natl Acad. Sci. USA 106, 14379–14384 (2009).
Fu, L. et al. Fibroblast growth factor 19 increases metabolic rate and reverses dietary and leptin-deficient diabetes. Endocrinology 145, 2594–2603 (2004).
Kong, B. et al. Mechanism of tissue-specific farnesoid X receptor in suppressing the expression of genes in bile-acid synthesis in mice. Hepatology 56, 1034–1043 (2012).
Li, S. et al. Cytoplasmic tyrosine phosphatase Shp2 coordinates hepatic regulation of bile acid and FGF15/19 signaling to repress bile acid synthesis. Cell. Metab. 20, 320–332 (2014).
Wang, C. et al. Hepatocyte FRS2α is essential for the endocrine fibroblast growth factor to limit the amplitude of bile acid production induced by prandial activity. Curr. Mol. Med. 14, 703–711 (2014).
Yu, C. et al. Elevated cholesterol metabolism and bile acid synthesis in mice lacking membrane tyrosine kinase receptor FGFR4. J. Biol. Chem. 275, 15482–15489 (2000).
Ito, S. et al. Impaired negative feedback suppression of bile acid synthesis in mice lacking βKlotho. J. Clin. Invest. 115, 2202–2208 (2005).
Tomiyama, K. et al. Relevant use of Klotho in FGF19 subfamily signaling system in vivo. Proc. Natl Acad. Sci. USA 107, 1666–1671 (2010).
Yu, C., Wang, F., Jin, C., Huang, X. & McKeehan, W. L. Independent repression of bile acid synthesis and activation of c-Jun N-terminal kinase (JNK) by activated hepatocyte fibroblast growth factor receptor 4 (FGFR4) and bile acids. J. Biol. Chem. 280, 17707–17714 (2005).
Kir, S., Zhang, Y., Gerard, R. D., Kliewer, S. A. & Mangelsdorf, D. J. Nuclear receptors HNF4α and LRH-1 cooperate in regulating Cyp7a1 in vivo. J. Biol. Chem. 287, 41334–41341 (2012).
Nitta, M., Ku, S., Brown, C., Okamoto, A. Y. & Shan, B. CPF: an orphan nuclear receptor that regulates liver-specific expression of the human cholesterol 7α-hydroxylase gene. Proc. Natl Acad. Sci. USA 96, 6660–6665 (1999).
Stroup, D. & Chiang, J. Y. HNF4 and COUP-TFII interact to modulate transcription of the cholesterol 7α-hydroxylase gene (CYP7A1). J. Lipid Res. 41, 1–11 (2000).
Miao, J. et al. Bile acid signaling pathways increase stability of small heterodimer partner (SHP) by inhibiting ubiquitin-proteasomal degradation. Genes Dev. 23, 986–996 (2009).
Lee, Y. K. et al. Liver receptor homolog-1 regulates bile acid homeostasis but is not essential for feedback regulation of bile acid synthesis. Mol. Endocrinol. 22, 1345–1356 (2008).
Potthoff, M. J., Kliewer, S. A. & Mangelsdorf, D. J. Endocrine fibroblast growth factors 15/19 and 21: from feast to famine. Genes Dev. 26, 312–324 (2012).
Tomlinson, E. et al. Transgenic mice expressing human fibroblast growth factor-19 display increased metabolic rate and decreased adiposity. Endocrinology 143, 1741–1747 (2002).
Wu, A. L. et al. FGF19 regulates cell proliferation, glucose and bile acid metabolism via FGFR4-dependent and independent pathways. PLoS ONE 6, e17868 (2011).
Yu, X. X. et al. Peripheral reduction of FGFR4 with antisense oligonucleotides increases metabolic rate and lowers adiposity in diet-induced obese mice. PLoS ONE 8, e66923 (2013).
Ge, H. et al. Fibroblast growth factor receptor 4 (FGFR4) deficiency improves insulin resistance and glucose metabolism under diet-induced obesity conditions. J. Biol. Chem. 289, 30470–30480 (2014).
Marcelin, G. et al. Central action of FGF19 reduces hypothalamic AGRP/NPY neuron activity and improves glucose metabolism. Mol. Metab. 3, 19–28 (2014).
Ryan, K. K. et al. Fibroblast growth factor-19 action in the brain reduces food intake and body weight and improves glucose tolerance in male rats. Endocrinology 154, 9–15 (2013).
Morton, G. J. et al. FGF19 action in the brain induces insulin-independent glucose lowering. J. Clin. Invest. 123, 4799–4808 (2013).
Katafuchi, T. et al. Detection of FGF15 in plasma by stable isotope standards and capture by anti-peptide antibodies and targeted mass spectrometry. Cell. Metab. 21, 898–904 (2015).
Lundasen, T., Galman, C., Angelin, B. & Rudling, M. Circulating intestinal fibroblast growth factor 19 has a pronounced diurnal variation and modulates hepatic bile acid synthesis in man. J. Intern. Med. 260, 530–536 (2006).
Schaap, F. G., van der Gaag, N. A., Gouma, D. J. & Jansen, P. L. High expression of the bile salt-homeostatic hormone fibroblast growth factor 19 in the liver of patients with extrahepatic cholestasis. Hepatology 49, 1228–1235 (2009).
Reiche, M. et al. Fibroblast growth factor 19 serum levels: relation to renal function and metabolic parameters. Horm. Metab. Res. 42, 178–181 (2010).
Lenicek, M. et al. Bile acid malabsorption in inflammatory bowel disease: assessment by serum markers. Inflamm. Bowel Dis. 17, 1322–1327 (2011).
Walters, J. R. et al. A new mechanism for bile acid diarrhea: defective feedback inhibition of bile acid biosynthesis. Clin. Gastroenterol. Hepatol 7, 1189–1194 (2009).
Schreuder, T. C. et al. The hepatic response to FGF19 is impaired in patients with nonalcoholic fatty liver disease and insulin resistance. Am. J. Physiol. Gastrointest. Liver Physiol. 298, G440–G445 (2010).
Mráz, M. et al. Serum concentrations of fibroblast growth factor 19 in patients with obesity and type 2 diabetes mellitus: the influence of acute hyperinsulinemia, very-low calorie diet and PPAR-α agonist treatment. Physiol. Res. 60, 627–636 (2011).
Nicholes, K. et al. A mouse model of hepatocellular carcinoma: ectopic expression of fibroblast growth factor 19 in skeletal muscle of transgenic mice. Am. J. Pathol. 160, 2295–2307 (2002).
Miura, S. et al. Fibroblast growth factor 19 expression correlates with tumor progression and poorer prognosis of hepatocellular carcinoma. BMC Cancer 12, 56 (2012).
Wu, X. et al. Separating mitogenic and metabolic activities of fibroblast growth factor 19 (FGF19). Proc. Natl Acad. Sci. USA 107, 14158–14163 (2010).
Sawey, E. T. et al. Identification of a therapeutic strategy targeting amplified FGF19 in liver cancer by oncogenomic screening. Cancer Cell 19, 347–358 (2011).
Hyeon, J., Ahn, S., Lee, J. J., Song, D. H. & Park, C. K. Expression of fibroblast growth factor 19 is associated with recurrence and poor prognosis of hepatocellular carcinoma. Dig. Dis. Sci. 58, 1916–1922 (2013).
Latasa, M. U. et al. Regulation of amphiregulin gene expression by β-catenin signaling in human hepatocellular carcinoma cells: a novel crosstalk between FGF19 and the EGFR system. PLoS ONE 7, e52711 (2012).
Uriarte, I. et al. Ileal FGF15 contributes to fibrosis-associated hepatocellular carcinoma development. Int. J. Cancer 136, 2469–2475 (2015).
Pai, R. et al. Inhibition of fibroblast growth factor 19 reduces tumor growth by modulating β-catenin signaling. Cancer Res. 68, 5086–5095 (2008).
French, D. M. et al. Targeting FGFR4 inhibits hepatocellular carcinoma in preclinical mouse models. PLoS ONE 7, e36713 (2012).
Desnoyers, L. R. et al. Targeting FGF19 inhibits tumor growth in colon cancer xenograft and FGF19 transgenic hepatocellular carcinoma models. Oncogene 27, 85–97 (2008).
Pai, R. et al. Antibody-mediated inhibition of fibroblast growth factor 19 results in increased bile acids synthesis and ileal malabsorption of bile acids in cynomolgus monkeys. Toxicol. Sci. 126, 446–456 (2012).
Hagel, M. et al. First selective small molecule inhibitor of FGFR4 for the treatment of hepatocellular carcinomas with an activated FGFR4 signaling pathway. Cancer Discov. 5, 424–437 (2015).
Wu, X. et al. C-terminal tail of FGF19 determines its specificity toward Klotho co-receptors. J. Biol. Chem. 283, 33304–33309 (2008).
Zhou, M. et al. Separating tumorigenicity from bile acid regulatory activity for endocrine hormone FGF19. Cancer Res. 74, 3306–3316 (2014).
Lundasen, T. et al. PPARα is a key regulator of hepatic FGF21. Biochem. Biophys. Res. Commun. 360, 437–440 (2007).
Patel, R. et al. Glucocorticoids regulate the metabolic hormone FGF21 in a feed-forward loop. Mol. Endocrinol. 29, 213–223 (2015).
Cyphert, H. A. et al. Activation of the farnesoid X receptor induces hepatic expression and secretion of fibroblast growth factor 21. J. Biol. Chem. 287, 25123–25138 (2012).
Li, Y., Wong, K., Walsh, K., Gao, B. & Zang, M. Retinoic acid receptor β stimulates hepatic induction of fibroblast growth factor 21 to promote fatty acid oxidation and control whole-body energy homeostasis in mice. J. Biol. Chem. 288, 10490–10504 (2013).
Adams, A. C. et al. Thyroid hormone regulates hepatic expression of fibroblast growth factor 21 in a PPARα-dependent manner. J. Biol. Chem. 285, 14078–14082 (2010).
Oishi, K. & Tomita, T. Thiazolidinediones are potent inducers of fibroblast growth factor 21 expression in the liver. Biol. Pharm. Bull. 34, 1120–1121 (2011).
Li, H. et al. Sodium butyrate stimulates expression of fibroblast growth factor 21 in liver by inhibition of histone deacetylase 3. Diabetes 61, 797–806 (2012).
Nygaard, E. B., Vienberg, S. G., Orskov, C., Hansen, H. S. & Andersen, B. Metformin stimulates FGF21 expression in primary hepatocytes. Exp. Diabetes Res. 2012, 465282 (2012).
Oishi, K., Konishi, M., Murata, Y. & Itoh, N. Time-imposed daily restricted feeding induces rhythmic expression of Fgf21 in white adipose tissue of mice. Biochem. Biophys. Res. Commun. 412, 396–400 (2011).
Dutchak, P. A. et al. Fibroblast growth factor-21 regulates PPARγ activity and the antidiabetic actions of thiazolidinediones. Cell 148, 556–567 (2012).
Wang, H., Qiang, L. & Farmer, S. R. Identification of a domain within peroxisome proliferator-activated receptor γ regulating expression of a group of genes containing fibroblast growth factor 21 that are selectively repressed by SIRT1 in adipocytes. Mol. Cell. Biol. 28, 188–200 (2008).
Hondares, E. et al. Thermogenic activation induces FGF21 expression and release in brown adipose tissue. J. Biol. Chem. 286, 12983–12990 (2011).
Fisher, F. M. et al. FGF21 regulates PGC-1α and browning of white adipose tissues in adaptive thermogenesis. Genes Dev. 26, 271–281 (2012).
Luo, Y. & McKeehan, W. L. Stressed liver and muscle call on adipocytes with FGF21. Front. Endocrinol. 4, 194 (2013).
Keipert, S. et al. Skeletal muscle mitochondrial uncoupling drives endocrine cross-talk through the induction of FGF21 as a myokine. Am. J. Physiol. Endocrinol. Metab. 306, E469–E482 (2014).
Adams, A. C. et al. The breadth of FGF21's metabolic actions are governed by FGFR1 in adipose tissue. Mol. Metab. 2, 31–37 (2012).
Foltz, I. N. et al. Treating diabetes and obesity with an FGF21-mimetic antibody activating the βKlotho/FGFR1c receptor complex. Sci Transl Med. 4, 162ra153 (2012).
Holland, W. L. et al. An FGF21-adiponectin-ceramide axis controls energy expenditure and insulin action in mice. Cell. Metab. 17, 790–797 (2013).
Lin, Z. et al. Adiponectin mediates the metabolic effects of FGF21 on glucose homeostasis and insulin sensitivity in mice. Cell. Metab. 17, 779–789 (2013).
Kersten, S. et al. Peroxisome proliferator-activated receptor α mediates the adaptive response to fasting. J. Clin. Invest. 103, 1489–1498 (1999).
Leone, T. C., Weinheimer, C. J. & Kelly, D. P. A critical role for the peroxisome proliferator-activated receptor α (PPARα) in the cellular fasting response: the PPARα-null mouse as a model of fatty acid oxidation disorders. Proc. Natl Acad. Sci. USA 96, 7473–7478 (1999).
Markan, K. R. et al. Circulating FGF21 is liver derived and enhances glucose uptake during refeeding and overfeeding. Diabetes 63, 4057–4063 (2014).
Berglund, E. D. et al. Fibroblast growth factor 21 controls glycemia via regulation of hepatic glucose flux and insulin sensitivity. Endocrinology 150, 4084–4093 (2009).
Emanuelli, B. et al. Interplay between FGF21 and insulin action in the liver regulates metabolism. J. Clin. Invest. 124, 515–527 (2014).
Bookout, A. L. et al. FGF21 regulates metabolism and circadian behavior by acting on the nervous system. Nat. Med. 19, 1147–1152 (2013).
Liang, Q. et al. FGF21 maintains glucose homeostasis by mediating the cross talk between liver and brain during prolonged fasting. Diabetes 63, 4064–4075 (2014). This is the first evidence that glucose homeostasis is maintained by fine tuning the inter-organ crosstalk between the liver and brain, and that FGF21 has a role in this setting.
Bell, G. I. and Polonsky, K. S. Diabetes mellitus and genetically programmed defects in β-cell function. Nature 414, 788–791 (2001).
So, W. Y. et al. High glucose represses β-klotho expression and impairs fibroblast growth factor 21 action in mouse pancreatic islets: involvement of peroxisome proliferator-activated receptor γ signaling. Diabetes 62, 3751–3759 (2013).
Kharitonenkov, A. et al. The metabolic state of diabetic monkeys is regulated by fibroblast growth factor-21. Endocrinology 148, 774–781 (2007).
So, W. Y., Cheng, G., Xu, A., Lam, K. S. L. & Leung, P. S. Loss of fibroblast growth factor 21 action induces insulin resistance, pancreatic islet hyperplasia and dysfunction in mice. Cell Death Dis. 6, e1707 (2015).
Johnson, C. L. et al. Fibroblast growth factor 21 reduces the severity of cerulein-induced pancreatitis in mice. Gastroenterology 137, 1795–1804 (2009).
Inagaki, T. et al. Inhibition of growth hormone signaling by the fasting-induced hormone FGF21. Cell. Metab. 8, 77–83 (2008).
Zhang, J. & Li, Y. Fibroblast growth factor 21, the endocrine FGF pathway and novel treatments for metabolic syndrome. Drug Discov. Today 19, 579–589 (2014).
Zhang, Y. et al. The starvation hormone, fibroblast growth factor-21, extends lifespan in mice. eLife 1, e00065 (2012).
De Sousa-Coelho, A. L. et al. FGF21 mediates the lipid metabolism response to amino acid starvation. J. Lipid Res. 54, 1786–1797 (2013).
Laeger, T. et al. FGF21 is an endocrine signal of protein restriction. J. Clin. Invest. 124, 3913–3922 (2014).
Ahmadian, M. et al. PPARγ signaling and metabolism: the good, the bad and the future. Nat. Med. 19, 557–566 (2013).
Adams, A. C. et al. Fibroblast growth factor 21 is not required for the antidiabetic actions of the thiazoladinediones. Mol. Metab. 2, 205–214 (2013).
Wei, W. et al. Fibroblast growth factor 21 promotes bone loss by potentiating the effects of peroxisome proliferator-activated receptor γ. Proc. Natl Acad. Sci. USA 109, 3143–3148 (2012).
Veniant, M. M. et al. Pharmacological effects of FGF21 are independent of the 'browning' of white adipose tissue. Cell. Metab. 21, 731–738 (2015).
Samms, R. J. et al. Discrete aspects of FGF21 in vivo pharmacology do not require UCP1. Cell. Reports 11, 991–999 (2015).
Lee, P., Swarbrick, M. M. & Greenfield, J. R. The sum of all browning in FGF21 therapeutics. Cell. Metab. 21, 795–796 (2015).
Owen, B. M., Mangelsdorf, D. J. & Kliewer, S. A. Tissue-specific actions of the metabolic hormones FGF15/19 and FGF21. Trends Endocrinol. Metab. 26, 22–29 (2015).
Sarruf, D. A. et al. Fibroblast growth factor 21 action in the brain increases energy expenditure and insulin sensitivity in obese rats. Diabetes 59, 1817–1824 (2010).
Owen, B. M. et al. FGF21 contributes to neuroendocrine control of female reproduction. Nat. Med. 19, 1153–1156 (2013).
Owen, B. M. et al. FGF21 acts centrally to induce sympathetic nerve activity, energy expenditure, and weight loss. Cell. Metab. 20, 670–677 (2014).
Yan, X. et al. FGF21 deletion exacerbates diabetic cardiomyopathy by aggravating cardiac lipid accumulation. J. Cell. Mol. Med. 19, 1557–1568 (2015).
Planavila, A. et al. Fibroblast growth factor 21 protects against cardiac hypertrophy in mice. Nat. Commun. 4, 2019 (2013).
Planavila, A. et al. Fibroblast growth factor 21 protects the heart from oxidative stress. Cardiovasc. Res. 106, 19–31 (2015).
Liu, S. Q. et al. Endocrine protection of ischemic myocardium by FGF21 from the liver and adipose tissue. Sci. Rep. 3, 2767 (2013).
Galman, C. et al. The circulating metabolic regulator FGF21 is induced by prolonged fasting and PPARα activation in man. Cell. Metab. 8, 169–174 (2008).
Oishi, K., Uchida, D. & Ishida, N. Circadian expression of FGF21 is induced by PPARα activation in the mouse liver. FEBS Lett. 582, 3639–3642 (2008).
Andersen, B., Beck-Nielsen, H. & Hojlund, K. Plasma FGF21 displays a circadian rhythm during a 72-h fast in healthy female volunteers. Clin. Endocrinol. 75, 514–519 (2011).
Yu, H. et al. Circadian rhythm of circulating fibroblast growth factor 21 is related to diurnal changes in fatty acids in humans. Clin. Chem. 57, 691–700 (2011).
Lee, S. A. et al. Various oscillation patterns of serum fibroblast growth factor 21 concentrations in healthy volunteers. Diabetes Metab. J. 36, 29–36 (2012).
Christodoulides, C., Dyson, P., Sprecher, D., Tsintzas, K. & Karpe, F. Circulating fibroblast growth factor 21 is induced by peroxisome proliferator-activated receptor agonists but not ketosis in man. J. Clin. Endocrinol. Metab. 94, 3594–3601 (2009).
Dushay, J. et al. Increased fibroblast growth factor 21 in obesity and nonalcoholic fatty liver disease. Gastroenterology 139, 456–463 (2010).
Mraz, M. et al. Serum concentrations and tissue expression of a novel endocrine regulator fibroblast growth factor-21 in patients with type 2 diabetes and obesity. Clin. Endocrinol. 71, 369–375 (2009).
Hondares, E. et al. Fibroblast growth factor-21 is expressed in neonatal and pheochromocytoma-induced adult human brown adipose tissue. Metabolism 63, 312–317 (2014).
Lee, P. et al. Irisin and FGF21 are cold-induced endocrine activators of brown fat function in humans. Cell. Metab. 19, 302–309 (2014).
Lin, Z. et al. Serum levels of FGF-21 are increased in coronary heart disease patients and are independently associated with adverse lipid profile. PLoS ONE 5, e15534 (2010).
Dostalova, I. et al. Plasma concentrations of fibroblast growth factors 19 and 21 in patients with anorexia nervosa. J. Clin. Endocrinol. Metab. 93, 3627–3632 (2008).
Kharitonenkov, A. & Adams, A. C. Inventing new medicines: the FGF21 story. Mol. Metab. 3, 221–229 (2014).
Woo, Y. C., Xu, A., Wang, Y. & Lam, K. S. Fibroblast growth factor 21 as an emerging metabolic regulator: clinical perspectives. Clin. Endocrinol. 78, 489–496 (2013).
Adams, A. C. et al. LY2405319, an engineered FGF21 variant, improves the metabolic status of diabetic monkeys. PLoS ONE 8, e65763 (2013).
Veniant, M. M. et al. Long-acting FGF21 has enhanced efficacy in diet-induced obese mice and in obese rhesus monkeys. Endocrinology 153, 4192–4203 (2012).
Nygaard, E. B., Moller, C. L., Kievit, P., Grove, K. L. & Andersen, B. Increased fibroblast growth factor 21 expression in high-fat diet-sensitive non-human primates (Macaca mulatta). Int. J. Obes 38, 183–191 (2014).
Fisher, F. M. et al. Obesity is a fibroblast growth factor 21 (FGF21)-resistant state. Diabetes 59, 2781–2789 (2010).
Gimeno, R. E. & Moller, D. E. FGF21-based pharmacotherapy — potential utility for metabolic disorders. Trends Endocrinol. Metab. 25, 303–311 (2014).
Hecht, R. et al. Rationale-based engineering of a potent long-acting FGF21 analog for the treatment of type 2 diabetes. PLoS ONE 7, e49345 (2012).
Kharitonenkov, A. et al. Rational design of a fibroblast growth factor 21-based clinical candidate, LY2405319. PLoS ONE 8, e58575 (2013).
Mu, J. et al. FGF21 analogs of sustained action enabled by orthogonal biosynthesis demonstrate enhanced antidiabetic pharmacology in rodents. Diabetes 61, 505–512 (2012).
Xu, J. et al. Polyethylene glycol modified FGF21 engineered to maximize potency and minimize vacuole formation. Bioconjug. Chem. 24, 915–925 (2013).
Huang, Z. et al. A better anti-diabetic recombinant human fibroblast growth factor 21 (rhFGF21) modified with polyethylene glycol. PLoS ONE 6, e20669 (2011).
Camacho, R. C., Zafian, P. T., Achanfuo-Yeboah, J., Manibusan, A. & Berger, J. P. Pegylated Fgf21 rapidly normalizes insulin-stimulated glucose utilization in diet-induced insulin resistant mice. Eur. J. Pharmacol. 715, 41–45 (2013).
Song, L. et al. A solid-phase PEGylation strategy for protein therapeutics using a potent FGF21 analog. Biomaterials 35, 5206–5215 (2014).
Huang, J. et al. Development of a novel long-acting antidiabetic FGF21 mimetic by targeted conjugation to a scaffold antibody. J. Pharmacol. Exp. Ther. 346, 270–280 (2013).
Weng, Y. et al. Pharmacokinetics (PK), pharmacodynamics (PD) and integrated PK/PD modeling of a novel long acting FGF21 clinical candidate PF-05231023 in diet-induced obese and leptin-deficient obese mice. PLoS ONE 10, e0119104 (2015).
Giragossian, C. et al. Mechanistic investigation of the pre-clinical pharmacokinetics and interspecies scaling of PF-05231023, a fibroblast growth factor 21-antibody protein conjugate. Drug Metab. Dispos. 43, 803–811 (2015).
Doppalapudi, V. R. et al. Chemical generation of bispecific antibodies. Proc. Natl Acad. Sci. USA 107, 22611–22616 (2010).
Wu, A. L. et al. Amelioration of type 2 diabetes by antibody-mediated activation of fibroblast growth factor receptor 1. Sci Transl. Med. 3, 113ra126 (2011).
Smith, R. et al. FGF21 can be mimicked in vitro and in vivo by a novel anti-FGFR1c/β-Klotho bispecific protein. PLoS ONE 8, e61432 (2013).
Kolek, O. I. et al. 1α,25-dihydroxyvitamin D3 upregulates FGF23 gene expression in bone: the final link in a renal-gastrointestinal-skeletal axis that controls phosphate transport. Am. J. Physiol. Gastrointest. Liver Physiol. 289, G1036–G1042 (2005).
Yu, X., Sabbagh, Y., Davis, S. I., Demay, M. B. & White, K. E. Genetic dissection of phosphate- and vitamin D-mediated regulation of circulating Fgf23 concentrations. Bone 36, 971–977 (2005).
Shimada, T. et al. FGF-23 transgenic mice demonstrate hypophosphatemic rickets with reduced expression of sodium phosphate cotransporter type IIa. Biochem. Biophys. Res. Commun. 314, 409–414 (2004).
Meir, T. et al. Parathyroid hormone activates the orphan nuclear receptor Nurr1 to induce FGF23 transcription. Kidney Int. 86, 1106–1115 (2014).
Lanske, B. & Razzaque, M. S. Molecular interactions of FGF23 and PTH in phosphate regulation. Kidney Int. 86, 1072–1074 (2014).
Lavi-Moshayoff, V., Wasserman, G., Meir, T., Silver, J. & Naveh-Many, T. PTH increases FGF23 gene expression and mediates the high-FGF23 levels of experimental kidney failure: a bone parathyroid feedback loop. Am. J. Physiol. Renal Physiol. 299, F882–F889 (2010).
Ben-Dov, I. Z. et al. The parathyroid is a target organ for FGF23 in rats. J. Clin. Invest. 117, 4003–4008 (2007).
Galitzer, H., Ben-Dov, I., Lavi-Moshayoff, V., Naveh-Many, T. & Silver, J. Fibroblast growth factor 23 acts on the parathyroid to decrease parathyroid hormone secretion. Curr. Opin. Nephrol. Hypertens. 17, 363–367 (2008).
Shimada, T. et al. Vitamin D receptor-independent FGF23 actions in regulating phosphate and vitamin D metabolism. Am. J. Physiol. Renal Physiol. 289, F1088–F1095 (2005).
Tsuji, K., Maeda, T., Kawane, T., Matsunuma, A. & Horiuchi, N. Leptin stimulates fibroblast growth factor 23 expression in bone and suppresses renal 1α,25-dihydroxyvitamin D3 synthesis in leptin-deficient ob/ob mice. J. Bone Miner. Res. 25, 1711–1723 (2010).
Haussler, M. R. et al. The role of vitamin D in the FGF23, klotho, and phosphate bone-kidney endocrine axis. Rev. Endocr. Metab. Disord. 13, 57–69 (2012).
Shimada, T. et al. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J. Bone Miner. Res. 19, 429–435 (2004).
Kuro-o, M. et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 390, 45–51 (1997). This is the first paper to describe the discovery of α-klotho.
Drüeke, T. B. & Prié, D. Klotho spins the thread of life — what does klotho do to the receptor of fibroblast growth factor-23 (FGF23)? Nephrol. Dial. Transplant 22, 1524–1526 (2007).
Farrow, E. G. & White, K. E. Recent advances in renal phosphate handling. Nat. Rev. Nephrol. 6, 207–217 (2010).
Hu, M. C. et al. Klotho: a novel phosphaturic substance acting as an autocrine enzyme in the renal proximal tubule. FASEB J. 24, 3438–3450 (2010).
Andrukhova, O. et al. FGF23 acts directly on renal proximal tubules to induce phosphaturia through activation of the ERK1/2-SGK1 signaling pathway. Bone 51, 621–628 (2012).
Razzaque, M. S. & Lanske, B. Hypervitaminosis D and premature aging: lessons learned from Fgf23 and Klotho mutant mice. Trends Mol. Med. 12, 298–305 (2006).
Hesse, M., Fröhlich, L. F., Zeitz, U., Lanske, B. & Erben, R. G. Ablation of vitamin D signaling rescues bone, mineral, and glucose homeostasis in Fgf-23 deficient mice. Matrix Biol. 26, 75–84 (2007).
Ohnishi, M., Nakatani, T., Lanske, B. & Razzaque, M. S. Reversal of mineral ion homeostasis and soft-tissue calcification of klotho knockout mice by deletion of vitamin D 1α-hydroxylase. Kidney Int. 75, 1166–1172 (2009).
Barthel, T. K. et al. 1,25-Dihydroxyvitamin D3/VDR-mediated induction of FGF23 as well as transcriptional control of other bone anabolic and catabolic genes that orchestrate the regulation of phosphate and calcium mineral metabolism. J. Steroid Biochem. Mol. Biol. 103, 381–388 (2007).
Bai, X., Miao, D., Li, J., Goltzman, D. & Karaplis, A. C. Transgenic mice overexpressing human fibroblast growth factor 23 (R176Q) delineate a putative role for parathyroid hormone in renal phosphate wasting disorders. Endocrinology 145, 5269–5279 (2004).
Gutiérrez, O. M. et al. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N. Engl. J. Med. 359, 584–592 (2008).
Galitzer, H., Ben-Dov, I. Z., Silver, J. & Naveh-Many, T. Parathyroid cell resistance to fibroblast growth factor 23 in secondary hyperparathyroidism of chronic kidney disease. Kidney Int. 77, 211–218 (2010).
Komaba, H. et al. Depressed expression of Klotho and FGF receptor 1 in hyperplastic parathyroid glands from uremic patients. Kidney Int. 77, 232–238 (2010).
Hofman-Bang, J., Martuseviciene, G., Santini, M. A., Olgaard, K. & Lewin, E. Increased parathyroid expression of klotho in uremic rats. Kidney Int. 78, 1119–1127 (2010).
Mirza, M. A. et al. Relationship between circulating FGF23 and total body atherosclerosis in the community. Nephrol. Dial. Transplant 24, 3125–3131 (2009).
Mirza, M. A., Larsson, A., Melhus, H., Lind, L. & Larsson, T. E. Serum intact FGF23 associate with left ventricular mass, hypertrophy and geometry in an elderly population. Atherosclerosis 207, 546–551 (2009).
Gutiérrez, O. M. et al. Fibroblast growth factor 23 and left ventricular hypertrophy in chronic kidney disease. Circulation 119, 2545–2552 (2009).
Faul, C. et al. FGF23 induces left ventricular hypertrophy. J. Clin. Invest. 121, 4393–4408 (2011).
Touchberry, C. D. et al. FGF23 is a novel regulator of intracellular calcium and cardiac contractility in addition to cardiac hypertrophy. Am. J. Physiol. Endocrinol. Metab. 304, E863–E873 (2013).
Jonsson, K. B. et al. Fibroblast growth factor 23 in oncogenic osteomalacia and X-linked hypophosphatemia. N. Engl. J. Med. 348, 1656–1663 (2003).
Yamazaki, Y. et al. Increased circulatory level of biologically active full-length FGF-23 in patients with hypophosphatemic rickets/osteomalacia. J. Clin. Endocrinol. Metab. 87, 4957–4960 (2002).
Imel, E. A., Hui, S. L. & Econs, M. J. FGF23 concentrations vary with disease status in autosomal dominant hypophosphatemic rickets. J. Bone Miner. Res. 22, 520–526 (2007).
Mirza, M. A. et al. Relationship between circulating FGF23 and total body atherosclerosis in the community. Nephrol. Dial. Transplant 24, 3125–3131 (2009).
Quarles, L. D. Skeletal secretion of FGF-23 regulates phosphate and vitamin D metabolism. Nat. Rev. Endocrinol. 8, 276–286 (2012).
Olauson, H. et al. Targeted deletion of Klotho in kidney distal tubule disrupts mineral metabolism. J. Am. Soc. Nephrol. 23, 1641–1651 (2012).
Quarles, L. D. The bone and beyond: 'Dem bones' are made for more than walking. Nat. Med. 17, 428–430 (2011).
Yamazaki, Y. et al. Anti-FGF23 neutralizing antibodies show the physiological role and structural features of FGF23. J. Bone Miner. Res. 23, 1509–1518 (2008).
Aono, Y. et al. Therapeutic effects of anti-FGF23 antibodies in hypophosphatemic rickets/osteomalacia. J. Bone Miner. Res. 24, 1879–1888 (2009).
Carpenter, T. O. et al. Randomized trial of the anti-FGF23 antibody KRN23 in X-linked hypophosphatemia. J. Clin. Invest. 124, 1587–1597 (2014).
Shalhoub, V. et al. FGF23 neutralization improves chronic kidney disease-associated hyperparathyroidism yet increases mortality. J. Clin. Invest. 122, 2543–2553 (2012).
Koizumi, M., Komaba, H., Nakanishi, S., Fujimori, A. & Fukagawa, M. Cinacalcet treatment and serum FGF23 levels in haemodialysis patients with secondary hyperparathyroidism. Nephrol. Dial. Transplant 27, 784–790 (2012).
Gonzalez-Parra, E. et al. Lanthanum carbonate reduces FGF23 in chronic kidney disease Stage 3 patients. Nephrol. Dial. Transplant 26, 2567–2571 (2011).
Di Marco, G. S. et al. Treatment of established left ventricular hypertrophy with fibroblast growth factor receptor blockade in an animal model of CKD. Nephrol. Dial. Transplant 29, 2028–2035 (2014).
Wu, A. L. et al. Antibody-mediated activation of FGFR1 induces FGF23 production and hypophosphatemia. PLoS ONE 8, e57322 (2013).
Muller, T. D. et al. Restoration of leptin responsiveness in diet-induced obese mice using an optimized leptin analog in combination with exendin-4 or FGF21. J. Pept. Sci. 18, 383–393 (2012).
Acknowledgements
The authors apologize to our distinguished colleagues whose work has not been cited owing to space limitations. The authors are indebted to Dr R. Le Donne for the artwork. The authors received research grants from the European Marie Curie Initial Training Network (NR-NET 606806), the Italian Association for Cancer Research (AIRC, IG 14732), the Italian Ministry of University and Education (Finanziamenti per la Ricerca di Base IDEAS RBID08C9N7; PRIN 2010FHH32M-002), the Italian Ministry of Health (Young Researchers Grant GR-2010-2314703), Intercept Pharmaceuticals (San Diego CA), NGM BioPharmaceuticals (San Francisco CA) and University of Bari Aldo Moro (IDEA GRBA0802SJ-2008).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Heparin or heparan sulphate glycosaminoglycans
-
(HSGAGs). Long chains of repeated saccharide units that can be variably sulphated or acetylated, allowing for considerable structural diversity. HSGAGs are located in the extracellular matrix of the surface of every cell, where they modulate the activity of a wide range of growth factors and morphogens.
- Tumour-induced osteomalacia
-
(TIO). A rare paraneoplastic syndrome in which patients present with bone pain, fractures and muscle weakness. This uncommon disorder results in increased renal phosphate excretion, hypophosphataemia and osteomalacia.
- Cholestasis
-
A decrease in bile flow owing to impaired secretion by hepatocytes or owing to obstruction of bile flow through intra-or extrahepatic bile ducts. Therefore, the clinical definition of cholestasis is any condition in which substances that are normally excreted into bile are retained.
- Promoter
-
An element of DNA that may bind RNA polymerase and other proteins for the successful initiation of transcription directly upstream of the gene.
- Insulin resistance
-
The inability of a known quantity of exogenous or endogenous insulin to induce a normal increase in glucose uptake and utilization. Insulin resistance occurs as part of a cluster of cardiovascular-metabolic abnormalities commonly referred to as the insulin resistance syndrome or the metabolic syndrome.
- Non-alcoholic fatty liver disease
-
(NAFLD). A condition defined by excessive fat accumulation in the form of triglycerides (steatosis) in the liver. In the majority of patients, NAFLD is associated with metabolic risk factors such as obesity, diabetes mellitus, and dyslipidaemia.
- Hypothalamic–pituitary–adrenal axis
-
(HPA axis). A complex set of direct influences and feedback interactions among three endocrine glands: the hypothalamus, the pituitary gland and the adrenal glands. The HPA axis controls reactions to stress and regulates many body processes, including digestion, the immune system, mood and emotions, sexuality, and energy storage and expenditure.
- Sumoylation
-
A post-translational modification process affecting protein structure and subcellular localization. It is analogous to ubiquitylation in terms of the reaction scheme and enzyme classes used, but rather than conjugation by ubiquitin, sumoylation involves addition of SUMOs (small ubiquitin-like modifiers).
- Suprachiasmatic nucleus
-
(SCN). A tiny region located in the hypothalamus, situated directly above the optic chiasm, responsible for controlling circadian rhythms. The neuronal and hormonal activities it generates regulate many different body functions in a 24-hour cycle, using around 20,000 neurons. The SCN contains several cell types and several different peptides (including vasopressin and vasoactive intestinal peptide) and neurotransmitters.
- Dorsal vagal complex
-
(DVC). The DVC encompasses the nucleus tractus solitarii (NTS), the dorsal motor nucleus of the vagus nerve (DMX) and the area postrema (AP), that altogether provide the major integrative centre for the mammalian autonomic nervous system.
- Hyperparathyroidism
-
A disease characterized by excessive secretion of parathyroid hormone, an 84-amino-acid polypeptide hormone.
Rights and permissions
About this article
Cite this article
Degirolamo, C., Sabbà, C. & Moschetta, A. Therapeutic potential of the endocrine fibroblast growth factors FGF19, FGF21 and FGF23. Nat Rev Drug Discov 15, 51–69 (2016). https://doi.org/10.1038/nrd.2015.9
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrd.2015.9
This article is cited by
-
Heating-mediated purification of active FGF21 and structure-based design of its variant with enhanced potency
Scientific Reports (2023)
-
Treatment with fibroblast growth factor 19 increases skeletal muscle fiber size, ameliorates metabolic perturbations and hepatic inflammation in 5/6 nephrectomized mice
Scientific Reports (2023)
-
Sex difference on fibroblast growth factors (FGFs) expression in skin and wound of streptozotocin(STZ)-induced type 1 diabetic mice
Molecular Biology Reports (2023)
-
Bile acid signaling in the regulation of whole body metabolic and immunological homeostasis
Science China Life Sciences (2023)
-
Hepatokines: the missing link in the development of insulin resistance and hyperandrogenism in PCOS?
Hormones (2023)