Key Points
-
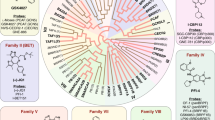
Mammalian BET proteins, a class of transcriptional co-regulators that contain dual, mutually related bromodomain motifs and an extraterminal domain, are important in the control of networks of genes; these proteins bind to acetylated lysines in the histones of nucleosomal chromatin, recruit chromatin-modification enzymes to target promoters and function as co-activators or co-repressors of gene expression, depending on the context.
-
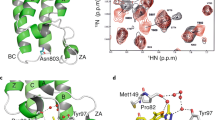
New small-molecule inhibitors have recently been developed that disrupt the binding interface between the bromodomain and the acetylated lysine groups; the inhibitors have remarkable potency, selectivity and are well tolerated. They have recently been used as anticancer and anti-inflammatory agents.
-
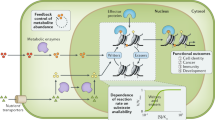
These developments are important because chromatin was not considered to be a druggable target; as a result of these new drugs, a whole field of new epigenetically targeted therapeutics has become available for investigation.
-
As this field of therapeutics rapidly expands, several features of BET protein function will need to be considered, including possible redundancy among the closely related family members, the selectivity of next-generation agents for specific BET proteins, and possible undesirable consequences of systemic administration without cellular targeting. These side effects might include uncontrolled transcriptional derepression of genes, altered haematopoiesis, immunosuppression or reactivation of latent viruses.
Abstract
The bromodomain is a highly conserved motif of 110 amino acids that is bundled into four anti-parallel α-helices and found in proteins that interact with chromatin, such as transcription factors, histone acetylases and nucleosome remodelling complexes. Bromodomain proteins are chromatin 'readers'; they recruit chromatin-regulating enzymes, including 'writers' and 'erasers' of histone modification, to target promoters and to regulate gene expression. Conventional wisdom held that complexes involved in chromatin dynamics are not 'druggable' targets. However, small molecules that inhibit bromodomain and extraterminal (BET) proteins have been described. We examine these developments and discuss the implications for small molecule epigenetic targeting of chromatin networks in cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Kubonishi, I. et al. Novel t(15;19)(q15;p13) chromosome abnormality in a thymic carcinoma. Cancer Res. 51, 3327–3328 (1991).
French, C. A. et al. BRD4-NUT fusion oncogene: a novel mechanism in aggressive carcinoma. Cancer Res. 63, 304–307 (2003).
French, C. A. et al. BRD-NUT oncoproteins: a family of closely related nuclear proteins that block epithelial differentiation and maintain the growth of carcinoma cells. Oncogene 27, 2237–2242 (2008).
Florence, B. & Faller, D. V. You bet-cha: a novel family of transcriptional regulators. Front. Biosci. 6, D1008–D1018 (2001).
Wu, S. Y. & Chiang, C. M. The double bromodomain-containing chromatin adaptor Brd4 and transcriptional regulation. J. Biol. Chem. 282, 13141–13145 (2007).
Haynes, S. R. et al. The bromodomain: a conserved sequence found in human, Drosophila and yeast proteins. Nucl. Acids Res. 20, 2603 (1992). This report offered the first suggestion that the bromodomain module is widely conserved and is likely to have a role in chromatin status and transcriptional regulation in diverse organisms.
Jeanmougin, F., Wurtz, J.-M., Le Douarin, B., Chambon, P. & Losson, R. The bromodomain revisited. Trends Biochem. Sci. 22, 151–153 (1997).
Winston, F. & Allis, C. D. The bromodomain: a chromatin-targeting module? Nature Struct. Biol. 6, 601–604 (1999).
Horn, P. J. & Peterson, C. L. The bromodomain: a regulator of ATP-dependent chromatin remodeling? Front. Biosci. 6, D1019–1023 (2001).
Jiang, Y. W. et al. Mammalian mediator of transcriptional regulation and its possible role as an end-point of signal transduction pathways. Proc. Natl Acad. Sci. USA 95, 8538–8543 (1998).
Kuras, L., Borggrefe, T. & Kornberg, R. D. Association of the Mediator complex with enhancers of active genes. Proc. Natl Acad. Sci. USA 100, 13887–13891 (2003).
Kornberg, R. D. Mediator and the mechanism of transcriptional activation. Trends Biochem. Sci. 30, 235–239 (2005).
Dhalluin, C. et al. Structure and ligand of a histone acetyltransferase bromodomain. Nature 399, 491–496 (1999).
Sanchez, R. & Zhou, M.-M. The role of human bromodomains in chromatin biology and gene transcription. Curr. Opin. Drug Discov. Dev. 12, 659–665 (2009).
Tamkun, J. W. et al. brahma – a regulator of Drosophila homeotic genes structurally related to the yeast transcriptional activator SWI2/SNF2. Cell 68, 561–572 (1992).
Wang, W. et al. Purification and biochemical heterogeneity of the mammalian SWI-SNF complex. EMBO J. 15, 5370–5382 (1996).
Pazin, M. J. & Kadonaga, J. T. SWI2/SNF2 and related proteins: ATP-driven motors that disrupt protein-DNA interactions? Cell 88, 737–740 (1997).
Ogryzko, V. V., Schiltz, O. L., Russanova, V., Howard, B. H. & Nakatani, Y. The transcriptional co-activators p300 and CBP are histone acetyltransferases. Cell 87, 953–959 (1996).
Bannister, A. J. & Kouzarides, T. The CBP co-activator is a histone acetyltransferase. Nature 384, 641–643 (1996).
Beck, S., Hanson, I., Kelly, A., Pappin, D. J. C. & Trowsdale, J. A homologue of the Drosophila female sterile homeotic (fsh) gene in the class II region of the human MHC. DNA Seq. 2, 203–210 (1992).
Matangkasombut, O., Buratowski, R. M., Swilling, N. W. & Buratowski, S. Bromodomain factor 1 corresponds to the missing piece of yeast TFIID. Genes Dev. 14, 951–962 (2000).
Thorpe, K. L., Abdulla, S., Kaufman, J., Trowsdale, J. & Beck, S. Phylogeny and structure of the RING3 gene. Immunogenet. 44, 391–396 (1996).
Salter-Cid, L., Pasquier, L. & Flajnik, M. RING3 is linked to the Xenopus major histocompatibility complex. Immunogenet. 44, 397–399 (1996).
Takami, K., Zaleska-Rutczynska, Z., Figueroa, F. & Klein, J. Linkage of LMP, TAP, and RING3 with Mhc class I rather than class II genes in the zebrafish. J. Immunol. 159, 6052–6060 (1997).
Deshaies, R. J. & Joazeiro, C. A. P. RING domain E3 ubiquitin ligases. Ann. Rev. Biochem. 78, 399–434 (2009).
Ting, J. P. & Trowsdale, J. Genetic control of MHC class II expression. Cell 109, S21–33 (2002).
Denis, G. V. & Green, M. R. A novel, mitogen-activated nuclear kinase is related to a Drosophila developmental regulator. Genes Dev. 10, 261–271 (1996). This was the first report of a BET protein phenotype in mammalian cells.
Guo, N., Faller, D. V. & Denis, G. V. Activation-induced nuclear translocation of RING3. J. Cell Sci. 113, 3085–3091 (2000).
Denis, G. V., Vaziri, C., Guo, N. & Faller, D. V. RING3 kinase transactivates promoters of cell cycle regulatory genes through E2F. Cell Growth Diff. 11, 417–424 (2000).
Ostrowski, J., Florio, S. K., Denis, G. V., Suzuki, H. & Bomsztyk, K. Stimulation of p85/RING3 kinase in multiple organs after systemic administration of mitogens into mice. Oncogene 16, 1223–1227 (1998).
Crowley, T. E., Kaine, E. M., Yoshida, M., Nandi, A. & Wolgemuth, D. J. Reproductive cycle regulation of nuclear import, euchromatic localization, and association with components of Pol. II mediator of a mammalian double-bromodomain protein. Mol. Endocrinol. 16, 1727–1737 (2002).
Kanno, T. et al. Selective recognition of acetylated histones by bromodomain proteins visualized in living cells. Mol. Cell. 13, 33–43 (2004).
Nakamura, Y. et al. Crystal structure of the human BRD2 bromodomain: insights into dimerization and recognition of acetylated histone H4. J. Biol. Chem. 282, 4193–4201 (2007).
Denis, G. V. et al. Identification of transcription complexes that contain the double bromodomain protein Brd2 and chromatin remodeling machines. J. Proteome Res. 5, 502–511 (2006).
LeRoy, G., Rickards, B. & Flint, S. J. The double bromodomain proteins Brd2 and Brd3 couple histone acetylation to transcription. Mol. Cell. 30, 51–60 (2008).
Denis, G. V. Bromodomain motifs and “scaffolding”? Front. Biosci. 6, D1066–D1068 (2001).
Peng, J. et al. Brd2 is a TBP-associated protein and recruits TBP into E2F-1 transcriptional complex in response to serum stimulation. Mol. Cell. Biochem. 294, 45–54 (2007).
Sinha, A., Faller, D. V. & Denis, G. V. Bromodomain analysis of Brd2-dependent transcriptional activation of cyclin A. Biochem. J. 387, 257–269 (2005).
Lin, Y. J. et al. Solution structure of the extraterminal domain of the bromodomain-containing protein BRD4. Protein Sci. 17, 2174–2179 (2008).
Rahman, S. et al. The Brd4 extraterminal domain confers transcription activation independent of pTEFb by recruiting multiple proteins, including NSD3. Mol. Cell. Biol. 31, 2641–2652 (2011).
Bartkova, J. et al. Cyclin D1 protein expression and function in human breast cancer. Int. J. Cancer 57, 353–361 (1994).
Courjal, F. et al. Mapping of DNA amplifications at 15 chromosomal localizations in 1875 breast tumors: Definition of phenotypic groups. Cancer Res. 57, 4360c4367 (1997).
Ormandy, C. J. et al. Cyclin D1, EMS1 and 11q13 amplification in breast cancer. Breast Cancer Res. Treat. 78, 323–335 (2003).
Jang, M. K. et al. The bromodomain protein Brd4 is a positive regulatory component of P-TEFb and stimulates RNA polymerase II-dependent transcription. Mol. Cell. 19, 523–534 (2005).
Yang, Z. et al. Recruitment of P-TEFb for stimulation of transcriptional elongation by the bromodomain protein Brd4. Mol. Cell. 19, 535–545 (2005).
Marshall, N. F., Peng, J., Xie, Z. & Price, D. H. Control of RNA polymerase II elongation potential by a novel carboxyl-terminal domain kinase. J. Biol. Chem. 271, 27176–27183 (1996).
Yang, Z., He, N. & Zhou, Q. Brd4 recruits P-TEFb to chromosomes at late mitosis to promote G1 gene expression and cell cycle progression. Mol. Cell. Biol. 28, 967–976 (2008).
Maruyama, T. et al. A Mammalian bromodomain protein, Brd4, interacts with replication factor C and inhibits progression to S phase. Mol. Cell. Biol. 22, 6509–6520 (2002).
Dey, A., Chitsaz, F., Abbasi, A., Misteli, T. & Ozato, K. The double bromodomain protein Brd4 binds to acetylated chromatin during interphase and mitosis. Proc. Natl Acad. Sci. USA. 100, 8758–8763 (2003).
Zhang, L. et al. microRNA-141 is involved in a nasopharyngeal carcinoma-related genes network. Carcinogenesis 31, 559–566 (2010).
Dey, A. et al. A bromodomain protein, MCAP, associates with mitotic chromosomes and affects G(2)-to-M transition. Mol. Cell. Biol. 20, 6537–6549 (2000).
Umehara, T. et al. Structural basis for acetylated histone H4 recognition by the human BRD2 bromodomain. J. Biol. Chem. 285, 7610–7618 (2010).
Nishiyama, A., Dey, A., Miyazaki, J. & Ozato, K. Brd4 is required for recovery from antimicrotubule drug-induced mitotic arrest: preservation of acetylated chromatin. Mol. Biol. Cell. 17, 814–823 (2006).
Laue, K. et al. The multidomain protein Brpf1 binds histones and is required for Hox gene expression and segmental identity. Development 135, 1935–1946 (2008).
Toyama, R., Rebbert, M. L., Dey, A. & Ozato, K. & Dawid, I. B. Brd4 associates with mitotic chromosomes throughout early zebrafish embryogenesis. Dev. Dyn. 237, 1636–1644 (2008).
Dey, A., Nishiyama, A., Karpova, T., McNally, J. & Ozato, K. Brd4 marks select genes on mitotic chromatin and directs postmitotic transcription. Mol. Biol. Cell 20, 4899–4909 (2009).
Chua, P. & Roeder, G. S. Bdf1, a yeast chromosomal protein required for sporulation. Mol. Cell. Biol. 15, 3685–3696 (1995).
Della Rovere, F. et al. The Arabidopsis BET bromodomain factor GTE4 regulates the mitotic cell cycle. Plant Signal Behav. 5, 677–680 (2010).
Airoldi, C. A. et al. The Arabidopsis BET bromodomain factor GTE4 is involved in maintenance of the mitotic cell cycle during plant development. Plant Physiol. 152, 1320–1334 (2010).
Shibata, Y., Takeshita, H., Sasakawa, N. & Sawa, H. Double bromodomain protein BET-1 and MYST HATs establish and maintain stable cell fates in C. elegans. Development 137, 1045–1053 (2010).
You, J. et al. Kaposi's Sarcoma-associated herpesvirus latency-associated nuclear antigen interacts with bromodomain protein Brd4 on host mitotic chromosomes. J. Virol. 80, 8909–8919 (2006).
Zhao, R., Nakamura, T., Fu, Y., Lazar, Z. & Spector, D. L. Gene bookmarking accelerates the kinetics of post-mitotic transcriptional re-activation. Nature Cell Biol. 13, 1295–1304 (2011).
Gyuris, A. et al. The chromatin-targeting protein Brd2 is required for neural tube closure and embryogenesis. Biochim. Biophys. Acta. 1789, 413–421 (2009).
Shang, E., Wang, X., Wen, D., Greenberg, D. A. & Wolgemuth, D. J. Double bromodomain-containing gene Brd2 is essential for embryonic development in mouse. Dev. Dyn. 238, 908–917 (2009).
Wang, F. et al. Brd2 disruption in mice causes severe obesity without type 2 diabetes. Biochem. J. 425, 71–83 (2009). References 63–65 established that Brd2 -null mice are not viable. Reference 65 reports the surprising finding of low-inflammatory, metabolically protected obesity in Brd2 -hypomorphic mice.
Houzelstein, D. et al. Growth and early postimplantation defects in mice deficient for the bromodomain-containing protein Brd4. Mol. Cell. Biol. 22, 3794–3802 (2002). Along with references 48 and 49, this report established that Brd4 -null mice are not viable owing to cell cycle defects.
You, J. et al. Regulation of aurora B expression by the bromodomain protein Brd4. Mol. Cell. Biol. 29, 5094–5103 (2009).
Lygerou, Z. et al. The yeast BDF1 gene encodes a transcription factor involved in the expression of a broad class of genes including snRNAs. Nucl. Acids Res. 22, 5332–5340 (1994).
Matangkasombut, O. & Buratowski, S. Different sensitivities of bromodomain factors 1 and 2 to histone H4 acetylation. Mol. Cell 11, 353–363 (2003).
Jacobson, R. H., Ladurner, A. G., King, D. S. & Tjian, R. Structure and function of a human TAFII250 double bromodomain module. Science 288, 1422–1425 (2000).
Dunphy, E. L., Johnson, T., Auerbach, S. S. & Wang, E. H. Requirement for TAF(II)250 acetyltransferase activity in cell cycle progression. Mol. Cell. Biol. 20, 1134–1139 (2000).
Wang, E. H., Zou, S. & Tjian, R. TAFII250-dependent transcription of cyclin A is directed by ATF activator proteins. Genes Dev. 11, 2658–2669 (1997).
Iacobuzio-Donahue, C. A. Epigenetic changes in cancer. Ann. Rev. Pathol. 4, 229–249 (2009).
Redner, R. L., Wang, J. & Liu, J. M. Chromatin remodeling and leukaemia: new therapeutic paradigms. Blood 94, 417–428 (1999).
Borrow, J. et al. The translocation t(8;16)(p11;p13) of acute myeloid leukaemia fuses a putative acetyltransferase to the CREB-binding protein. Nature Genet. 14, 33–41 (1996).
Sobulo, O. M. et al. MLL is fused to CBP, a histone acetyltransferase, in therapy-related acute myeloid leukaemia with a t(11;16)(q23;p13.3). Proc. Natl Acad. Sci. USA 94, 8732–8737 (1997).
Lavau, C., Du, C., Thirman, M. & Zeleznik-Le, N. Chromatin-related properties of CBP fused to MLL generate a myelodysplastic-like syndrome that evolves into myeloid leukaemia. EMBO J. 19, 4655–4664 (2000).
Martens, J. H. et al. PML-RARalpha/RXR alters the epigenetic landscape in Acute Promyelocytic Leukaemia. Cancer Cell 17, 173–185 (2010).
Filippakopoulos, P. et al. Selective inhibition of BET bromodomains. Nature 468, 1067–1073 (2010). First report of small-molecule inhibition of bromodomain proteins that demonstrated that epigenetically acting therapeutics that disrupt chromatin–co-activator interactions are feasible and have therapeutic benefit.
Muller, S., Filippakopoulos, P. & Knapp, S. Bromodomains as therapeutic targets. Expert Rev. Mol. Med. 13, e29 (2011).
Mertz, J. A. et al. Targeting MYC dependence in cancer by inhibiting BET bromodomains. Proc. Natl Acad. Sci. USA 108, 16669–16674 (2011).
Seoane, J., Le, H. V. & Massagué, J. Myc suppression of the p21(Cip1) Cdk inhibitor influences the outcome of the p53 response to DNA damage. Nature 419, 729–734 (2002).
Boxer, L. M. & Dang, C. V. Translocations involving c-myc and c-myc function. Oncogene 20, 5595–5610 (2001).
Zuber, J. et al. RNAi screen identifies Brd4 as a therapeutic target in acute myeloid leukaemia. Nature 478, 524–528 (2011).
Ciro, M. et al. ATAD2 is a novel cofactor for MYC, overexpressed and amplified in aggressive tumors. Cancer Res. 69, 8491–8498 (2009).
Caron, C. et al. Functional characterization of ATAD2 as a new cancer/testis factor and a predictor of poor prognosis in breast and lung cancers. Oncogene 29, 5171–5181 (2010).
Soucek, L. et al. Modelling Myc inhibition as a cancer therapy. Nature 455, 679–683 (2008).
Soucek, L., Nasi, S. & Evan, G. I. Omomyc expression in skin prevents Myc-induced papillomatosis. Cell Death Differ. 11, 1038–1045 (2004).
Centeno, F. Ramírez-Salazar, E., García-Villa, E., Gariglio, P. & Garrido, E. TAF1 interacts with and modulates human papillomavirus 16 E2-dependent transcriptional regulation. Intervirology 51, 137–143 (2008).
Zhou, M. et al. Bromodomain protein Brd4 regulates human immunodeficiency virus transcription through phosphorylation of CDK9 at threonine 29. J. Virol. 83, 1036–1044 (2009).
Mueller, D. et al. A role for the MLL fusion partner ENL in transcriptional elongation and chromatin modification. Blood 110, 4445–4454 (2007).
Dawson, M. A. et al. Inhibition of BET recruitment to chromatin as an effective treatment for MLL-fusion leukaemia. Nature 478, 529–533 (2011). This article describes BET protein inhibitors in MLL, and confirms a hypothesis first proposed in reference 27.
Greenwald, R. J. et al. Eμ-BRD2 transgenic mice develop B cell lymphoma and leukaemia. Blood 103, 1475–1484 (2004).
Lenburg, M. E., Sinha, A., Faller, D. V. & Denis, G. V. Tumor-specific and proliferation-specific gene expression typifies murine transgenic B cell lymphomagenesis. J. Biol. Chem. 282, 4803–4811 (2007).
French, C. A. Pathogenesis of NUT midline carcinoma. Ann. Rev. Pathol. Mech. Dis. 7, 247–265 (2012).
Delmore, J. E. et al. BET bromodomain inhibition as a therapeutic strategy to target c-Myc. Cell 146, 904–917 (2011). This article describes MYC as a co-activator of BET proteins in human multiple myeloma and responsiveness to JQ1 therapeutics.
Nicodeme, E. et al. Suppression of inflammation by a synthetic histone mimic. Nature 468, 1119–1123 (2010). First demonstration that small-molecule BET protein inhibitors have potent and broad-spectrum anti-inflammatory properties.
Ouchida, R. et al. Suppression of NF-κB-dependent gene expression by a hexamethylene bisacetamide-inducible protein HEXIM1 in human vascular smooth muscle cells. Genes Cells 8, 95–107 (2003).
Hewings, D. S. et al. 3,5-dimethylisoxazoles act as acetyl-lysine-mimetic bromodomain ligands. J. Med. Chem. 54, 6761–6770 (2011).
Chung, C. W., Dean, A. W., Woolven, J. M. & Bamborough, P. Fragment-based discovery of bromodomain inhibitors part 1: inhibitor binding modes and implications for lead discovery. J. Med. Chem. 55, 576–586 (2012).
Bamborough, P. et al. Fragment-based discovery of bromodomain inhibitors part 2: optimization of phenylisoxazole sulfonamides. J. Med. Chem. 55, 587–596 (2012).
Belkina, A. C., Blanton, W., Wang, F., Liu, H. & Denis, G. V. Whole body Brd2 deficiency protects obese mice from insulin resistance by creating a low inflammatory environment. Obesity 18, S58 (2010).
Denis, G. V. Bromodomain coactivators in cancer, obesity, type 2 diabetes and inflammation. Discov. Med. 10, 489–499 (2010).
Andres, R. Effect of obesity on total mortality. Int. J. Obes. 4, 381–386 (1980).
Sims, E. A. H. Are there persons who are obese, but metabolically healthy? Metabolism 50, 1499–1504 (2001).
Klöting, N. et al. Insulin-sensitive obesity. Am. J. Physiol. Endocrinol. Metab. 299, E506–E515 (2010).
Bonora, E., et al. U-shaped and J-shaped relationships between serum insulin and coronary heart disease in the general population. The Bruneck Study. Diabetes Care 21, 221–230 (1991).
Wildman, R. P. et al. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: prevalence and correlates of 2 phenotypes among the US population Arch. Intern. Med. 168, 1617–1624 (2008).
Karelis, A. D. et al. The metabolically healthy but obese individual presents a favorable inflammation profile. J. Clin. Endocrinol. Metab. 90, 4145–4150 (2005).
Succurro, E. et al. Insulin secretion in metabolically obese, but normal weight, and in metabolically healthy but obese individuals. Obesity (Silver Spring) 16, 1881–1886 (2008).
Calori, G. et al. Prevalence, metabolic features, and prognosis of metabolically healthy obese Italian individuals: the Cremona Study. Diabetes Care 34, 210–215 (2011).
Huang, B., Yang, X. D., Zhou, M. M., Ozato, K. & Chen, L. F. Brd4 coactivates transcriptional activation of NF-kappaB via specific binding to acetylated RelA. Mol. Cell. Biol. 29, 1375–1387 (2009).
Cullen, S. J., Ponnappan, S. & Ponnappan, U. Catalytic activity of the proteasome finetunes Brg1-mediated chromatin remodeling to regulate the expression of inflammatory genes. Mol. Immunol. 47, 600–605 (2009).
Karin, M. NF-κB as a critical link between inflammation and cancer. Cold Spring Harb. Perspect. Biol. 1, a000141 (2009).
Virchow, R. Cellular Pathology, as Based on Physiological and Pathological Histology. (ed. Chance, F.) (J. Churchill, 1860).
Goldgraber, M. B., Humphreys, E. M., Kirsner, J. B. & Palmer, W. L. Carcinoma and ulcerative colitis, a clinical-pathologic study. II. Statistical analysis. Gastroenterology 34, 840–846 (1958).
Kraus, S. & Arber, N. Inflammation and colorectal cancer. Curr. Opin. Pharmacol. 9, 405–410 (2009).
Denis, G. V., Nikolajczyk, B. N. & Schnitzler, G. R. An emerging role for bromodomain-containing proteins in chromatin regulation and transcriptional control of adipogenesis. FEBS Lett. 584, 3260–3268 (2010).
Chung, C. W. et al. Discovery and characterization of small molecule inhibitors of the BET family bromodomains. J. Med. Chem. 54, 3827–3838 (2011).
Pamblanco, M. et al. Bromodomain factor 1 (Bdf1) protein interacts with histones. FEBS Lett. 496, 31–35 (2001).
Chang, Y. L., King, B., Lin, S. C., Kennison, J. A. & Huang, D. H. A double-bromodomain protein, FSH-S, activates the homeotic gene ultrabithorax through a critical promoter-proximal region. Mol. Cell. Biol. 27, 5486–5498 (2007).
Florence, B. L. & Faller, D. V. Drosophila female sterile (1) homeotic is a multifunctional transcriptional regulator that is modulated by Ras signaling. Dev. Dyn. 237, 554–564 (2008).
Denis, G. V. Duality in bromodomain-containing protein complexes. Front. Biosci. 6, D849–D852 (2001).
Trouche, D., Le Chalony, C., Muchardt, C., Yaniv, M. & Kouzarides, T. RB and hbrm cooperate to repress the activation functions of E2F1. Proc. Natl Acad. Sci. USA 94, 11268–11273 (1997).
Zhang, H. S. et al. Exit from G1 and S phase of the cell cycle is regulated by repressor complexes containing HDAC-Rb-hSWI/SNF and Rb-hSWI/SNF. Cell 101, 79–89 (2000).
Brehm, A. et al. Retinoblastoma protein recruits histone deacetylase to repress transcription. Nature 391, 597–601 (1998).
Ferreira, R., Magnaghi-Jaulin, L., Robin, P., Harel-Bellan, A. & Trouche, D. The three members of the pocket proteins family share the ability to repress E2F activity through recruitment of a histone deacetylase. Proc. Natl Acad. Sci. USA 95, 10493–10498 (1998).
Magnaghi-Jaulin, L. et al. Retinoblastoma protein represses transcription by recruiting a histone deacetylase. Nature 391, 601–605 (1998).
Stiegler, P., De Luca, A., Bagella, L. & Giordano, A. The COOH-terminal region of pRb2/p130 binds to histone deacetylase 1 (HDAC1), enhancing transcriptional repression of the E2F-dependent cyclin A promoter. Cancer Res. 58, 5049–5052 (1998).
Blobel, G. A., Kalota, A., Sanchez, P. V. & Carroll, M. Short hairpin RNA screen reveals bromodomain proteins as novel targets in acute myeloid leukaemia. Cancer Cell 20, 287–288 (2011).
Sachs, L. Control of normal cell differentiation in leukemic cells. Haematologica 61, 93–97 (1976).
Sachs, L. Leukemogenesis and differentiation. Ann. Rev. Med. 36, 177–184 (1985).
Dibenedetto, A. J. et al. Zebrafish brd2a and brd2b are paralogous members of the bromodomain-ET (BET) family of transcriptional coregulators that show structural and expression divergence. BMC Dev. Biol. 8, 39 (2008).
Sun, H. et al. Solution structure of BRD7 bromodomain and its interaction with acetylated peptides from histone H3 and H4. Biochem. Biophys. Res. Commun. 358, 435–441 (2007).
Tae, S. et al. Bromodomain protein 7 interacts with PRMT5 and PRC2, and is involved in transcriptional repression of their target genes. Nucleic Acids Res. 39, 5424–5438 (2011).
Ladurner, A. G., Inouye, C., Jain, R. & Tjian, R. Bromodomains mediate an acetyl-histone encoded antisilencing function at heterochromatin boundaries. Mol. Cell. 11, 365–376 (2003).
Hang, M. & Smith, M. M. Genetic analysis implicates the Set3/Hos2 histone deacetylase in the deposition and remodeling of nucleosomes containing H2AZ. Genetics 187, 1053–1066 (2011).
Look, A. T. Oncogenic transcription factors in the human acute leukaemias. Science 278, 1059–1064 (1997).
Rowley, J. D. The critical role of chromosome translocations in human leukaemias. Ann. Rev. Genet. 32, 495–519 (1998).
Wilson, B. G. & Roberts, C. W. M. SWI/SNF nucleosome remodelers and cancer. Nature Rev. Cancer 11, 481–492 (2011).
Cowell, I. G., Papageorgiou, N., Padget, K., Watters, G. P. & Austin, C. A. Histone deacetylase inhibition redistributes topoisomerase IIβ from heterochromatin to euchromatin. Nucleus 2, 61–71 (2011).
Gkikopoulos, T. et al. The SWI/SNF complex acts to constrain distribution of the centromeric histone variant Cse4. EMBO J. 30, 1919–1927 (2011).
Lamonica, J. M. et al. Bromodomain protein Brd3 associates with acetylated GATA1 to promote its chromatin occupancy at erythroid target genes. Proc. Natl Acad. Sci. USA 108, E159–E168 (2011). This report is one of the few that describes a function for the BET protein BRD3.
Gamsjaeger, R. et al. Structural basis and specificity of acetylated transcription factor GATA1 recognition by BET family bromodomain protein Brd3. Mol. Cell. Biol. 31, 2632–2640 (2011).
Haslam, D. W. & James, W. P. Obesity. Lancet 366, 1197–1209 (2009).
Hossain, P., Kawar, B. & El Nahas, M. Obesity and diabetes in the developing world--a growing challenge. N. Engl. J. Med. 356, 213–215 (2007).
Devaiah, B. N. et al. BRD4 is an atypical kinase that phosphorylates serine 2 of the RNA Polymerase II carboxy-terminal domain. Proc. Natl Acad. Sci. USA 109, 6927–6932 (2012).
Gans, M., Audit, C. & Masson, M. Isolation and characterization of sex-linked female-sterile mutants in Drosophila melanogaster. Genetics 81, 683–704 (1975).
Gans, M., Forquignon, F. & Masson, M. The role of dosage of the region 7D1-7D5-6 of the X chromosome in the production of homeotic transformations in Drosophila melanogaster. Genetics 96, 887–902 (1980).
Digan, M. E. et al. Genetic and molecular analysis of fs(1)h, a maternal effect homeotic gene in Drosophila. Dev. Biol. 114, 161–169 (1986).
Mozer, B. A. & Dawid, I. B. Cloning and molecular characterization of the trithorax locus of Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 86, 3738–3742 (1989).
Mazo, A. M., Huang, D. H., Mozer, B. A. & Dawid, I. B. The trithorax gene, a trans-acting regulator of the bithorax complex in Drosophila, encodes a protein with zinc-binding domains. Proc. Natl Acad. Sci. USA 87, 2112–2116 (1990). This report establishes that D. melanogaster BET proteins are activators of the Trithorax locus, and, with references 6 and 150, was the first described to describe functions for Metazoan bromodomain proteins.
Cimino, G. et al. Cloning of ALL-1, the locus involved in leukemias with the t(4;11)(q21;q23), t(9;11)(p22;q23), and t(11;19)(q23;p13) chromosome translocations. Cancer Res. 51, 6712–6714 (1991).
Djabali, M. et al. A trithorax-like gene is interrupted by chromosome 11q23 translocations in acute leukaemias. Nature Genet. 2, 113–118 (1992).
Gu, Y. et al. The t(4;11) chromosome translocation of human acute leukaemias fuses the ALL-1 gene, related to Drosophila trithorax, to the AF-4 gene. Cell 71, 701–708 (1992).
Tkachuk, D. C., Kohler, S. & Cleary, M. L. Involvement of a homolog of Drosophila trithorax by 11q23 chromosomal translocations in acute leukaemias. Cell 71, 691–700 (1992).
McPhillips, M. G., Ozato, K. & McBride, A. A. Interaction of bovine papillomavirus E2 protein with Brd4 stabilizes its association with chromatin. J. Virol. 79, 8920–8932 (2005).
Hebner, C. M. & Laimins, L. A. Human papillomaviruses: basic mechanisms of pathogenesis and oncogenicity. Rev. Med. Virol. 16, 83–97 (2006).
You, J., Croyle, J. L., Nishimura, A., Ozato, K. & Howley, P. M. Interaction of the bovine papillomavirus E2 protein with Brd4 tethers the viral DNA to host mitotic chromosomes. Cell 117, 349–360 (2004).
Schweiger, M. R., You, J. & Howley, P. M. Bromodomain protein 4 mediates the papillomavirus E2 transcriptional activation function. J. Virol. 80, 4276–4285 (2006).
McPhillips, M. G., Oliveira, J. G., Spindler, J. E., Mitra, R. & McBride, A. A. Brd4 is required for e2-mediated transcriptional activation but not genome partitioning of all papillomaviruses. J. Virol. 80, 9530–9543 (2006).
Senechal, H., Poirier, G. G., Coulombe, B., Laimins, L. A. & Archambault, J. Amino acid substitutions that specifically impair the transcriptional activity of papillomavirus E2 affect binding to the long isoform of Brd4. Virology 358, 10–17 (2007).
Wu, S. Y. et al. Brd4 links chromatin targeting to HPV transcriptional silencing. Genes Dev. 20, 2383–2396 (2006).
Smith, J. A. et al. Genome-wide siRNA screen identifies SMCX, EP400, and Brd4 as E2-dependent regulators of human papillomavirus oncogene expression. Proc. Natl Acad. Sci. USA 107, 3752–3757 (2010).
Ilves, I., Maemets, K., Silla, T., Janikson, K. & Ustav, M. Brd4 is involved in multiple processes of the bovine papillomavirus type 1 life cycle. J. Virol. 80, 3660–3665 (2006).
Platt, G. M., Simpson, G. R., Mittnacht, S. & Schulz, T. F. Latent nuclear antigen of Kaposi's sarcoma-associated herpesvirus interacts with RING3, a homolog of the Drosophila female sterile homeotic (fsh) gene. J. Virol. 73, 9789–9795 (1999).
Viejo-Borbolla, A. et al. Brd2/RING3 interacts with a chromatin-binding domain in the Kaposi's Sarcoma-associated herpesvirus latency-associated nuclear antigen 1 (LANA-1) that is required for multiple functions of LANA-1. J. Virol. 79, 13618–13629 (2005).
Ottinger, M. et al. Kaposi's sarcoma-associated herpesvirus LANA-1 interacts with the short variant of BRD4 and releases cells from a BRD4- and BRD2/RING3-induced G1 cell cycle arrest. J. Virol. 80, 10772–10786 (2006).
Radkov, S. A., Kellam, P. & Boshoff, C. The latent nuclear antigen of Kaposi sarcoma-associated herpesvirus targets the retinoblastoma-E2F pathway and with the oncogene Hras transforms primary rat cells. Nature Med. 6, 1121–1127 (2000).
Friborg, J. Jr, Kong, W., Hottiger, M. O. & Nabel, G. J. p53 inhibition by the LANA protein of KSHV protects against cell death. Nature 402, 889–894 (1999).
Palermo, R. D., Webb, H. M. & West, M. J. R. N. A. Polymerase II stalling promotes nucleosome occlusion and pTEFb recruitment to drive immortalization by Epstein-Barr Virus. PLoS Pathog. 7, e1002334 (2011). This was the first study to suggest that small-molecule BET protein inhibitors could have value in the treatment of latent virus infections by disrupting transcriptional co-regulator interactions.
Nagl, N. G. Jr, Zweitzig, D. R., Thimmapaya, B., Beck, G. R. Jr & Moran, E. The c-myc gene is a direct target of mammalian SWI/SNF-related complexes during differentiation associated cell cycle arrest. Cancer Res. 66, 1289–1293 (2006).
Nagl, N. G. Jr, Wang, X., Patsialou, A., Van Scoy, M. & Moran, E. Distinct mammalian SWI/SNF chromatin remodeling complexes with opposing roles in cell-cycle control. EMBO J. 26, 752–763 (2007).
Peterson, C. L. Chromatin remodeling: nucleosomes bulging at the seams. Curr. Biol. 12, R245–R247 (2002).
Sif, S., Saurin, A. J., Imbalzano, A. N. & Kingston, R. E. Purification and characterization of mSin3A-containing Brg1 and hBrm chromatin remodeling complexes. Genes Dev. 15, 603–618 (2001).
Olave, I., Wang, W., Xue, Y., Kuo, A. & Crabtree, G. R. Identification of a polymorphic, neuron-specific chromatin remodeling complex. Genes Dev. 16, 2509–2517 (2002).
Bultman, S. et al. A Brg1 null mutation in the mouse reveals functional differences among mammalian SWI/SNF complexes. Mol. Cell 6, 1287–1295 (2000).
Reyes, J. C. et al. Altered control of cellular proliferation in the absence of mammalian brahma (SNF2alpha). EMBO J. 17, 6979–6991 (1998).
Burns, L. G. & Peterson, C. L. The yeast SWI-SNF complex facilitates binding of a transcriptional activator to nucleosomal sites in vivo. Mol. Cell. Biol. 17, 4811–4819 (1997).
Holstege, F. C. et al. Dissecting the regulatory circuitry of a eukaryotic genome. Cell 95, 717–728 (1998). This report reveals an important principle that chromatin regulatory factors such as the Swi/Snf complex usually have dual functions as co-repressors and co-activators, implying that mutation or inhibition of their function will have pleiotropic effects. Combined with references 65 and 123, this principle suggests that JQ1, I-BET and related small-molecule inhibitors should be evaluated for the alleviation of co-repressor functions of BET proteins.
Chua, Y. L., Channeliere, S., Mott, E. & Gray, J. C. The bromodomain protein GTE6 controls leaf development in Arabidopsis by histone acetylation at ASYMMETRIC LEAVES1. Genes Dev. 19, 2245–2254 (2005).
Wang, R., Li, Q., Helfer, C. M., Jiao, J. & You, J. Bromodomain protein Brd4 associated with acetylated chromatin is important for maintenance of higher-order chromatin structure. J. Biol. Chem. 287, 10738–10752 (2012).
Acknowledgements
This work is supported by grants from the American Cancer Society, Leukaemia and Lymphoma Society and National Institutes of Health. The authors thank J. Bradner, B. Corkey, T. Gilmore, B. Nikolajczyk and M. Obin for useful discussions, and they thank their virology colleagues P. Howley, K. Kaye, D. Margolis, M. Montano and G. Viglianti for generously sharing reagents and ideas.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- Mediator transcription complex
-
Mediator is a large (1.2 MDa) multiprotein complex of up to 30 subunits that responds to signal transduction and that regulates transcription from a diverse set of RNA polymerase II-controlled promoters.
- Thienodiazepines
-
A chemical structural class built on a seven-membered 1,4-diazepine ring fused to a thiophene ring that provides a scaffold for derivatives of great current interest to medicinal chemistry because they target several pharmacologically important proteins, such as certain benzodiazepine derivatives that have neurological activity.
- Insulin resistance
-
A complex phenotype arising from reduced insulin responsiveness in tissues that transport glucose in response to insulin action, such as adipose tissue and skeletal muscle.
Rights and permissions
About this article
Cite this article
Belkina, A., Denis, G. BET domain co-regulators in obesity, inflammation and cancer. Nat Rev Cancer 12, 465–477 (2012). https://doi.org/10.1038/nrc3256
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrc3256
This article is cited by
-
Differential responses of pulmonary vascular cells from PAH patients and controls to TNFα and the effect of the BET inhibitor JQ1
Respiratory Research (2023)
-
Targeting bromodomain-containing proteins: research advances of drug discovery
Molecular Biomedicine (2023)
-
BRD4 is involved in viral exacerbation of chronic obstructive pulmonary disease
Respiratory Research (2023)
-
Bromodomain and extraterminal (BET) proteins: biological functions, diseases, and targeted therapy
Signal Transduction and Targeted Therapy (2023)
-
Role of non-coding RNAs in neuroblastoma
Cancer Gene Therapy (2023)