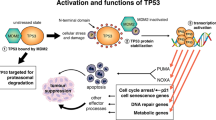
Key Points
-
The p53 pathway mediates innate tumour suppression in cells that have sustained genetic changes that drive tumour initiation and progression. p53 functions principally as a transcription factor that alters gene expression in favour of biological events, such as senescence or apoptosis, and the outcome of these events blocks the proliferation of or eliminates the tumour cell.
-
Early-stage human tumours show evidence of DNA damage, suggesting that this could be the signal by which p53 recognizes the incipient tumour. This notion is supported by the finding that oncogenes can induce DNA damage in cultured cells.
-
By contrast, some animal models show that induction of p53 in response to DNA damage has little protective effect against tumour formation. The induction of p53 by the ARF tumour suppressor pathway in these animals seems to be crucial for mediating p53-dependent tumour suppression.
-
p53 knock-in mice lacking key p53 phosphorylation sites that are modified through the DNA damage pathways but not through the ARF pathway show increased tumour susceptibility, but in a limited number of tissues. These mice provide evidence to support the idea that DNA damage pathways can, at least partially, influence tumour suppressor function.
Abstract
Loss of p53 function occurs during the development of most, if not all, tumour types. This paves the way for genomic instability, tumour-associated changes in metabolism, insensitivity to apoptotic signals, invasiveness and motility. However, the nature of the causal link between early tumorigenic events and the induction of the p53-mediated checkpoints that constitute a barrier to tumour progression remains uncertain. This Review considers the role of the DNA damage response, which is activated during the early stages of tumour development, in mobilizing the tumour suppression function of p53. The relationship between these events and oncogene-induced p53 activation through the ARF pathway is also discussed.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Lowe, S. W., Cepero, E. & Evan, G. Intrinsic tumour suppression. Nature 432, 307–315 (2004).
Vogelstein, B., Lane, D. & Levine, A. J. Surfing the p53 network. Nature 408, 307–310 (2000).
Vousden, K. H. & Lane, D. P. p53 in health and disease. Nature Rev. Mol. Cell Biol. 8, 275–283 (2007). This review provides a broad update on our understanding of p53 function, highlighting more recent discoveries that include the contribution of p53 under normal, unstressed, physiological conditions to processes, such as development, ageing and metabolism. It also discusses p53 as a target for therapeutic intervention.
Donehower, L. A. et al. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature 356, 215–221 (1992).
Harvey, M. et al. A mutant p53 transgene accelerates tumour development in heterozygous but not nullizygous p53-deficient mice. Nature Genet. 9, 305–311 (1995).
Iwakuma, T. & Lozano, G. Crippling p53 activities via knock-in mutations in mouse models. Oncogene 26, 2177–2184 (2007).
Lavigueur, A. et al. High incidence of lung, bone, and lymphoid tumors in transgenic mice overexpressing mutant alleles of the p53 oncogene. Mol. Cell. Biol. 9, 3982–3991 (1989).
Hainaut, P. & Hollstein, M. p53 and human cancer: the first ten thousand mutations. Adv. Cancer Res. 77, 81–137 (2000).
Danovi, D. et al. Amplification of Mdmx (or Mdm4) directly contributes to tumor formation by inhibiting p53 tumor suppressor activity. Mol. Cell. Biol. 24, 5835–5843 (2004).
Kamijo, T. et al. Functional and physical interactions of the ARF tumor suppressor with p53 and Mdm2. Proc. Natl Acad. Sci. USA 95, 8292–8297 (1998).
Onel, K. & Cordon-Cardo, C. MDM2 and prognosis. Mol. Cancer Res. 2, 1–8 (2004).
Sherr, C. J. The INK4a/ARF network in tumour suppression. Nature Rev. Mol. Cell. Biol. 2, 731–737 (2001).
Chen, Z. et al. Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature 436, 725–730 (2005).
Kemp, C. J., Donehower, L. A., Bradley, A. & Balmain, A. Reduction of p53 gene dosage does not increase initiation or promotion but enhances malignant progression of chemically induced skin tumors. Cell 74, 813–822 (1993).
Sands, A. T. et al. p53 deficiency does not affect the accumulation of point mutations in a transgene target. Proc. Natl Acad. Sci. USA 92, 8517–8521 (1995).
Ventura, A. et al. Restoration of p53 function leads to tumour regression in vivo. Nature 445, 661–665 (2007).
Xue, W. et al. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature 445, 656–660 (2007).
Campisi, J. & d'Adda di Fagagna, F. Cellular senescence: when bad things happen to good cells. Nature Rev. Mol. Cell Biol. 8, 729–740 (2007).
Sharpless, N. E. & DePinho, R. A. Cancer: crime and punishment. Nature 436, 636–637 (2005).
Bartkova, J. et al. Oncogene-induced senescence is part of the tumorigenesis barrier imposed by DNA damage checkpoints. Nature 444, 633–637 (2006). This paper provides direct evidence for oncogene-mediated DNA replication stress and shows that the DNA damage pathways are required for oncogene-dependent senescence in cultured cells.
Di Micco, R. et al. Oncogene-induced senescence is a DNA damage response triggered by DNA hyper-replication. Nature 444, 638–642 (2006).
White, E. & Lowe, S. W. Eating to exit: autophagy-enabled senescence revealed. Genes Dev. 23, 784–787 (2009).
Young, A. R. et al. Autophagy mediates the mitotic senescence transition. Genes Dev. 23, 798–803 (2009).
Schmitt, C. A. et al. Dissecting p53 tumor suppressor functions in vivo. Cancer Cell 1, 289–298 (2002).
Taylor, R. C., Cullen, S. P. & Martin, S. J. Apoptosis: controlled demolition at the cellular level. Nature Rev. Mol. Cell Biol. 9, 231–241 (2008).
Espinosa, J. M. Mechanisms of regulatory diversity within the p53 transcriptional network. Oncogene 27, 4013–4023 (2008).
Murray-Zmijewski, F., Slee, E. A. & Lu, X. A complex barcode underlies the heterogeneous response of p53 to stress. Nature Rev. Mol. Cell Biol. 9, 702–712 (2008).
Yonish-Rouach, E. et al. Wild-type p53 induces apoptosis of myeloid leukaemic cells that is inhibited by interleukin-6. Nature 352, 345–347 (1991).
Morgenbesser, S. D., Williams, B. O., Jacks, T. & DePinho, R. A. p53-dependent apoptosis produced by Rb-deficiency in the developing mouse lens. Nature 371, 72–74 (1994).
Qin, X. Q., Livingston, D. M., Kaelin, W. G. Jr & Adams, P. D. Deregulated transcription factor E2F-1 expression leads to S-phase entry and p53-mediated apoptosis. Proc. Natl Acad. Sci. USA 91, 10918–10922 (1994).
Godar, S. et al. Growth-inhibitory and tumor- suppressive functions of p53 depend on its repression of CD44 expression. Cell 134, 62–73 (2008). This paper shows that CD44, which is transcriptionally repressed by p53, is an essential tumour-promoting agent in cells that have lost p53 function.
Riley, T., Sontag, E., Chen, P. & Levine, A. Transcriptional control of human p53-regulated genes. Nature Rev. Mol. Cell Biol. 9, 402–412 (2008). This review provides a concise overview and analysis of the mechanisms of p53-mediated transcriptional regulation, defines criteria for p53-responsive genes and presents a comprehensive list of physiologically targeted p53-regulated genes.
Wei, C. L. et al. A global map of p53 transcription-factor binding sites in the human genome. Cell 124, 207–219 (2006).
Zhao, R. et al. Analysis of p53-regulated gene expression patterns using oligonucleotide arrays. Genes Dev. 14, 981–993 (2000).
Bensaad, K. et al. TIGAR, a p53-inducible regulator of glycolysis and apoptosis. Cell 126, 107–120 (2006).
Gatza, C., Moore, L., Dumble, M. & Donehower, L. A. Tumor suppressor dosage regulates stem cell dynamics during aging. Cell Cycle 6, 52–55 (2007).
Hu, W., Feng, Z., Atwal, G. S. & Levine, A. J. p53: a new player in reproduction. Cell Cycle 7, 848–852 (2008).
Hu, W., Feng, Z., Teresky, A. K. & Levine, A. J. p53 regulates maternal reproduction through LIF. Nature 450, 721–724 (2007).
Jones, R. G. et al. AMP-activated protein kinase induces a p53-dependent metabolic checkpoint. Mol. Cell 18, 283–293 (2005).
Kortlever, R. M., Higgins, P. J. & Bernards, R. Plasminogen activator inhibitor-1 is a critical downstream target of p53 in the induction of replicative senescence. Nature Cell Biol. 8, 877–884 (2006).
Matoba, S. et al. p53 regulates mitochondrial respiration. Science 312, 1650–1653 (2006).
Roger, L., Gadea, G. & Roux, P. Control of cell migration: a tumour suppressor function for p53? Biol. Cell 98, 141–152 (2006).
Stambolsky, P. et al. Regulation of AIF expression by p53. Cell Death Differ. 13, 2140–2149 (2006).
Teodoro, J. G., Evans, S. K. & Green, M. R. Inhibition of tumor angiogenesis by p53: a new role for the guardian of the genome. J. Mol. Med. 85, 1175–1186 (2007).
Wang, X. et al. p53 functions as a negative regulator of osteoblastogenesis, osteoblast-dependent osteoclastogenesis, and bone remodeling. J. Cell Biol. 172, 115–125 (2006).
Toledo, F. & Wahl, G. M. Regulating the p53 pathway: in vitro hypotheses, in vivo veritas. Nature Rev. Cancer 6, 909–923 (2006). This review presents a comprehensive picture of the molecular events through which p53 induction occurs in response to DNA damage, highlighting the roles of MDM2 and MDM4, and discussing evidence that argues against a key role for p53 modifications in this process.
Huang, L. C., Clarkin, K. C. & Wahl, G. M. Sensitivity and selectivity of the DNA damage sensor responsible for activating p53-dependent G1 arrest. Proc. Natl Acad. Sci. USA 93, 4827–4832 (1996).
Issaeva, N. et al. Small molecule RITA binds to p53, blocks p53–HDM-2 interaction and activates p53 function in tumors. Nature Med. 10, 1321–1328 (2004).
Vassilev, L. T. et al. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science 303, 844–848 (2004).
Yang, Y. et al. Small molecule inhibitors of HDM2 ubiquitin ligase activity stabilize and activate p53 in cells. Cancer Cell 7, 547–559 (2005).
Meulmeester, E. et al. Loss of HAUSP-mediated deubiquitination contributes to DNA damage-induced destabilization of Hdmx and Hdm2. Mol. Cell 18, 565–576 (2005).
Meulmeester, E., Pereg, Y., Shiloh, Y. & Jochemsen, A. G. ATM-mediated phosphorylations inhibit Mdmx/Mdm2 stabilization by HAUSP in favor of p53 activation. Cell Cycle 4, 1166–1170 (2005).
Stommel, J. M. & Wahl, G. M. Accelerated MDM2 auto-degradation induced by DNA-damage kinases is required for p53 activation. EMBO J. 23, 1547–1556 (2004).
Shiloh, Y. ATM and related protein kinases: safeguarding genome integrity. Nature Rev. Cancer 3, 155–168 (2003).
Anderson, C. W. & Appella, E. in Handbook of Cell Signaling (eds Bradshaw, R. A. & Dennis, E. A.) 237–247 (Elsevier, Amsterdam 2009).
Lowe, S. W. & Sherr, C. J. Tumor suppression by Ink4a–Arf: progress and puzzles. Curr. Opin. Genet. Dev. 13, 77–83 (2003).
de Stanchina, E. et al. E1A signaling to p53 involves the p19ARF tumor suppressor. Genes Dev. 12, 2434–2442 (1998).
Honda, R. & Yasuda, H. Association of p19ARF with Mdm2 inhibits ubiquitin ligase activity of Mdm2 for tumour suppressor p53. EMBO J. 18, 22–27 (1999).
Quelle, D. E., Zindy, F., Ashmunn, R. A. & Sherr, C. J. Alternative reading frames of the INK4A tumor suppressor gene encode two unrelated proteins capable of inducing cell cycle arrest. Cell 83, 993–1000 (1995).
Tao, W. & Levine, A. J. p19ARF stabilizes p53 by blocking nucleo-cytoplasmic shuttling of Mdm2. Proc. Natl Acad. Sci. USA 96, 6937–6941 (1999).
Weber, J. D., Taylor, L. J., Roussel, M. F., Sherr, C. J. & Bar-Sagi, D. Nucleolar ARF sequesters Mdm2 and activates p53. Nature Cell Biol. 1, 20–26 (1999).
Zhang, Y. & Xiong, Y. Mutations in human ARF exon 2 disrupt its nucleolar localization and impair its ability to block nuclear export of MDM2 and p53. Mol. Cell 3, 579–591 (1999).
Li, Y. et al. ATM activity contributes to the tumor-suppressing functions of p14ARF. Oncogene 23, 7355–7365 (2004).
Shieh, S.-Y., Ikeda, M., Taya, Y. & Prives, C. DNA damage-induced phosphorylation of p53 alleviates inhibition by MDM2. Cell 91, 325–334 (1997).
Siliciano, J. D. et al. DNA damage induces phosphorylation of the amino terminus of p53. Genes Dev. 11, 3471–3481 (1997).
Kamijo, T. et al. Tumor suppression at the mouse INK4A locus mediated by the alternative reading frame product p19ARF. Cell 91, 649–659 (1997).
Eischen, C. M., Weber, J. D., Roussel, M. F., Sherr, C. J. & Cleveland, J. L. Disruption of the ARF–Mdm2–p53 tumor suppressor pathway in Myc-induced lymphomagenesis. Genes Dev. 13, 2658–2669 (1999).
Schmitt, C. A., McCurrach, M. E., de Stanchina, E., Wallace-Brodeur, R. R. & Lowe, S. W. INK4a/ARF mutations accelerate lymphomagenesis and promote chemoresistance by disabling p53. Genes Dev. 13, 2670–2677 (1999).
Serrano, M. The INK4a/ARF locus in murine tumorigenesis. Carcinogenesis 21, 865–869 (2000).
Collins, C. J. & Sedivy, J. M. Involvement of the INK4a/Arf gene locus in senescence. Aging Cell 2, 145–150 (2003).
Ruas, M. & Peters, G. The p16INK4a/CDKN2A tumor suppressor and its relatives. Biochim. Biophys. Acta 1378, F115–177 (1998).
Bartkova, J. et al. DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature 434, 864–870 (2005).
Lindstrom, M. S. & Wiman, K. G. Myc and E2F1 induce p53 through p14ARF-independent mechanisms in human fibroblasts. Oncogene 22, 4993–5005 (2003).
DiTullio, R. A. Jr., et al. 53BP1 functions in an ATM-dependent checkpoint pathway that is constitutively activated in human cancer. Nature Cell Biol. 4, 998–1002 (2002).
Gorgoulis, V. G. et al. Activation of the DNA damage checkpoint and genomic instability in human precancerous lesions. Nature 434, 907–913 (2005). The analyses in this paper and in Reference 72 indicate that early precursor lesions in various tumour types show evidence of DNA damage and checkpoint activation, and are consistent with the idea that, from its earliest stages, cancer development is associated with DNA replication stress, which leads to DNA strand breaks.
d' Adda di Fagagna, F. Living on a break: cellular senescence as a DNA-damage response. Nature Rev. Cancer 8, 512–522 (2008).
Halazonetis, T. D., Gorgoulis, V. G. & Bartek, J. An oncogene-induced DNA damage model for cancer development. Science 319, 1352–1355 (2008).
Hirao, A. et al. Chk2 is a tumor suppressor that regulates apoptosis in both an ataxia–telangiectasia mutated (ATM)-dependent and an ATM-independent manner. Mol. Cell. Biol. 22, 6521–6532 (2002).
Ito, A. et al. p300/CBP-mediated p53 acetylation is commonly induced by p53-activating agents and inhibited by MDM2. EMBO J. 20, 1331–1340 (2001).
Mellert, H., Sykes, S. M., Murphy, M. E. & McMahon, S. B. The ARF/oncogene pathway activates p53 acetylation within the DNA binding domain. Cell Cycle 6, 1304–1306 (2007).
Armata, H. L., Garlick, D. S. & Sluss, H. K. The ataxia telangiectasia-mutated target site Ser18 is required for p53-mediated tumor suppression. Cancer Res. 67, 11696–11703 (2007).
MacPherson, D. et al. Defective apoptosis and B-cell lymphomas in mice with p53 point mutation at Ser 23. EMBO J. 23, 3689–3699 (2004).
Sluss, H. K., Armata, H., Gallant, J. & Jones, S. N. Phosphorylation of serine 18 regulates distinct p53 functions in mice. Mol. Cell. Biol. 24, 976–984 (2004).
Wu, Z. et al. Mutation of mouse p53 Ser23 and the response to DNA damage. Mol. Cell. Biol. 22, 2441–2449 (2002).
Chao, C., Herr, D., Chun, J. & Xu, Y. Ser18 and 23 phosphorylation is required for p53-dependent apoptosis and tumor suppression. EMBO J. 25, 2615–2622 (2006). This paper provides evidence that thymocytes from mice that harbour an alanine substitution of two key DNA damage-targeted phosphorylation sites (S18 and S23 (human S15 and S20)) in p53 are resistant to ionizing radiation-induced, p53-dependent apoptosis. The mice develop a range of malignancies, thereby establishing a link between a p53 modification mediated by DNA damage responses and tumour suppression.
Bruins, W. et al. Increased sensitivity to UV radiation in mice with a p53 point mutation at Ser389. Mol. Cell. Biol. 24, 8884–8894 (2004).
Hoogervorst, E. M. et al. Lack of p53 Ser389 phosphorylation predisposes mice to develop 2-acetylaminofluorene-induced bladder tumors but not ionizing radiation-induced lymphomas. Cancer Res. 65, 3610–3616 (2005).
Jeffers, J. R. et al. Puma is an essential mediator of p53-dependent and -independent apoptotic pathways. Cancer Cell 4, 321–328 (2003).
Bruins, W. et al. The absence of Ser389 phosphorylation in p53 affects the basal gene expression level of many p53-dependent genes and alters the biphasic response to UV exposure in mouse embryonic fibroblasts. Mol. Cell. Biol. 28, 1974–1987 (2008).
Christophorou, M. A., Ringshausen, I., Finch, A. J., Swigart, L. B. & Evan, G. I. The pathological response to DNA damage does not contribute to p53-mediated tumour suppression. Nature 443, 214–217 (2006). This paper shows that although p53 induced by whole-body ionizing radiation gives rise to widespread apoptosis, it does not offer any protection against lymphomagenesis. By contrast, the delay of p53 induction following genotoxic injury allows substantial protection against lymphoma development, which is ARF dependent.
Efeyan, A., Garcia-Cao, I., Herranz, D., Velasco-Miguel, S. & Serrano, M. Tumour biology: policing of oncogene activity by p53. Nature 443, 159 (2006).
Garcia-Cao, I. et al. “Super p53” mice exhibit enhanced DNA damage response, are tumor resistant and age normally. EMBO J. 21, 6225–6235 (2002).
Khan, S. H., Moritsugu, J. & Wahl, G. M. Differential requirement for p19ARF in the p53-dependent arrest induced by DNA damage, microtubule disruption, and ribonucleotide depletion. Proc. Natl Acad. Sci. USA 97, 3266–3271 (2000). This study describes a detailed analysis of the role of ARF in the p53 response to various stresses. The data indicate that ARF-null MEFs are partially defective in the DNA damage response owing to reduced levels of induced p53. In addition, this study shows that ARF expression is induced following treatment with ionizing radiation.
Eymin, B. et al. p14ARF activates a Tip60-dependent and p53-independent ATM/ATR/CHK pathway in response to genotoxic stress. Mol. Cell. Biol. 26, 4339–4350 (2006).
Tolbert, D., Lu, X., Yin, C., Tantama, M. & Van Dyke, T. p19ARF is dispensable for oncogenic stress-induced p53-mediated apoptosis and tumor suppression in vivo. Mol. Cell. Biol. 22, 370–377 (2002).
Wetmore, C., Eberhart, D. E. & Curran, T. Loss of p53 but not ARF accelerates medulloblastoma in mice heterozygous for patched. Cancer Res. 61, 513–516 (2001).
Moore, L. et al. Cooperativity of p19ARF, Mdm2, and p53 in murine tumorigenesis. Oncogene 22, 7831–7837 (2003).
Weber, J. D. et al. p53-independent functions of the p19ARF tumor suppressor. Genes Dev. 14, 2358–2365 (2000).
Wright, E. G. & Coates, P. J. Untargeted effects of ionizing radiation: implications for radiation pathology. Mutat. Res. 597, 119–132 (2006).
Wei, W., Hemmer, R. M. & Sedivy, J. M. Role of p14ARF in replicative and induced senescence of human fibroblasts. Mol. Cell. Biol. 21, 6748–6757 (2001).
Brown, E. J. & Baltimore, D. ATR disruption leads to chromosomal fragmentation and early embryonic lethality. Genes Dev. 14, 397–402 (2000).
O'Driscoll, M., Ruiz-Perez, V. L., Woods, C. G., Jeggo, P. A. & Goodship, J. A. A splicing mutation affecting expression of ataxia–telangiectasia and Rad3-related protein (ATR) results in Seckel syndrome. Nature Genet. 33, 497–501 (2003).
Moll, U. M. & Slade, N. p63 and p73: roles in development and tumor formation. Mol. Cancer Res. 2, 371–386 (2004).
Deyoung, M. P. & Ellisen, L. W. p63 and p73 in human cancer: defining the network. Oncogene 26, 5169–5183 (2007).
Flores, E. R. et al. p63 and p73 are required for p53-dependent apoptosis in response to DNA damage. Nature 416, 560–564 (2002).
Senoo, M., Manis, J. P., Alt, F. W. & McKeon, F. p63 and p73 are not required for the development and p53-dependent apoptosis of T cells. Cancer Cell 6, 85–89 (2004).
Suh, E. K. et al. p63 protects the female germ line during meiotic arrest. Nature 444, 624–628 (2006).
Costanzo, A. et al. DNA damage-dependent acetylation of p73 dictates the selective activation of apoptotic target genes. Mol. Cell 9, 175–186 (2002).
Flores, E. R. et al. Tumor predisposition in mice mutant for p63 and p73: evidence for broader tumor suppressor functions for the p53 family. Cancer Cell 7, 363–373 (2005).
Gong, J. G. et al. The tyrosine kinase c-Abl regulates p73 in apoptotic response to cisplatin-induced DNA damage. Nature 399, 806–809 (1999).
Pediconi, N. et al. Differential regulation of E2F1 apoptotic target genes in response to DNA damage. Nature Cell Biol. 5, 552–558 (2003).
Strano, S. et al. The transcriptional coactivator Yes-associated protein drives p73 gene-target specificity in response to DNA damage. Mol. Cell 18, 447–459 (2005).
Adorno, M. et al. A mutant-p53/Smad complex opposes p63 to empower TGFβ-induced metastasis. Cell 137, 87–98 (2009).
Clohessy, J. G. & Pandolfi, P. P. β-tting on p63 as a metastatic suppressor. Cell 137, 28–30 (2009).
Brooks, C. L. & Gu, W. p53 ubiquitination: Mdm2 and beyond. Mol. Cell 21, 307–315 (2006).
Jones, S. N., Roe, A. E., Donehower, L. A. & Bradley, A. Rescue of embryonic lethality in Mdm2-deficient mice by absence of p53. Nature 378, 206–208 (1995).
Montes de Oca Luna, R., Wagner, D. S. & Lozano, G. Rescue of early embryonic lethality in mdm2-deficient mice by deletion of p53. Nature 378, 203–206 (1995).
Ringshausen, I., O'Shea, C. C., Finch, A. J., Swigart, L. B. & Evan, G. I. Mdm2 is critically and continuously required to suppress lethal p53 activity in vivo. Cancer Cell 10, 501–514 (2006). This paper shows the universal requirement for MDM2 in controlling p53 function in vivo , provides evidence that a robust p53 response can be achieved simply by uncoupling the p53–MDM2 association and shows that unregulated p53 induction can give rise to fatal systemic pathologies.
Wu, X., Bayle, J. H., Olson, D. & Levine, A. J. The p53—mdm-2 autoregulatory feedback loop. Genes Dev. 7, 1126–1132 (1993).
Marine, J. C. et al. Keeping p53 in check: essential and synergistic functions of Mdm2 and Mdm4. Cell Death Differ. 13, 927–934 (2006).
Toledo, F. & Wahl, G. M. MDM2 and MDM4: p53 regulators as targets in anticancer therapy. Int. J. Biochem. Cell Biol. 39, 1476–1482 (2007).
Chen, L., Gilkes, D. M., Pan, Y., Lane, W. S. & Chen, J. ATM and Chk2-dependent phosphorylation of MDMX contribute to p53 activation after DNA damage. EMBO J. 24, 3411–3422 (2005).
Fang, S., Jensen, J. P., Ludwig, R. L., Vousden, K. H. & Weissman, A. M. Mdm2 is a RING finger-dependent ubiquitin protein ligase for itself and p53. J. Biol. Chem. 275, 8945–8951 (2000).
Honda, R. & Yasuda, H. Activity of MDM2, a ubiquitin ligase, toward p53 or itself is dependent on the RING finger domain of the ligase. Oncogene 19, 1473–1476 (2000).
Pereg, Y. et al. Phosphorylation of Hdmx mediates its Hdm2- and ATM-dependent degradation in response to DNA damage. Proc. Natl Acad. Sci. USA 102, 5056–5061 (2005).
Feng, J. et al. Stabilization of Mdm2 via decreased ubiquitination is mediated by protein kinase B/Akt-dependent phosphorylation. J. Biol. Chem. 279, 35510–35517 (2004).
Francoz, S. et al. Mdm4 and Mdm2 cooperate to inhibit p53 activity in proliferating and quiescent cells in vivo. Proc. Natl Acad. Sci. USA 103, 3232–3237 (2006).
Li, M., Brooks, C. L., Kon, N. & Gu, W. A dynamic role of HAUSP in the p53-Mdm2 pathway. Mol. Cell 13, 879–886 (2004).
Tang, J. et al. Critical role for Daxx in regulating Mdm2. Nature Cell Biol. 8, 855–862 (2006). This paper shows that the adaptor protein DAXX normally targets the deubiquitylating enzyme HAUSP towards Mdm2, thereby minimizing MDM2-mediated self-ubiquitylation and destruction. However, after DNA damage, DAXX dissociates from MDM2, leading to self-degradation of MDM2.
Toledo, F. et al. A mouse p53 mutant lacking the proline-rich domain rescues Mdm4 deficiency and provides insight into the Mdm2–Mdm4–p53 regulatory network. Cancer Cell 9, 273–285 (2006).
Sheng, Y. et al. Molecular recognition of p53 and MDM2 by USP7/HAUSP. Nature Struct. Mol. Biol. 13, 285–291 (2006).
Kulikov, R., Winter, M. & Blattner, C. Binding of p53 to the central domain of Mdm2 is regulated by phosphorylation. J. Biol. Chem. 281, 28575–28583 (2006).
Shimizu, H. et al. The conformationally flexible S9–S10 linker region in the core domain of p53 contains a novel MDM2 binding site whose mutation increases ubiquitination of p53 in vivo. J. Biol. Chem. 277, 28446–28458 (2002).
Wallace, M., Worrall, E., Pettersson, S., Hupp, T. R. & Ball, K. L. Dual-site regulation of MDM2 E3-ubiquitin ligase activity. Mol. Cell 23, 251–263 (2006).
Yu, G. W. et al. The central region of HDM2 provides a second binding site for p53. Proc. Natl Acad. Sci. USA 103, 1227–1232 (2006).
Banin, S. et al. Enhanced phosphorylation of p53 by ATM in response to DNA damage. Science 281, 1674–1677 (1998).
Canman, C. E. et al. Activation of the ATM kinase by ionising radiation and phosphorylation of p53. Science 281, 1677–1679 (1998).
Khanna, K. K. et al. ATM associates with and phosphorylates p53: mapping the region of interaction. Nature Genet. 20, 398–400 (1998).
Lakin, N. D., Hann, B. C. & Jackson, S. P. The ataxia–telangiectasia related protein ATR mediates DNA-dependent phosphorylation of p53. Oncogene 18, 3989–3995 (1999).
Dumaz, N., Milne, D. M. & Meek, D. W. Protein kinase CK1 is a p53 threonine-18 protein kinase which requires prior phosphorylation of serine 15. FEBS Lett. 463, 312–316 (1999).
Sakaguchi, K. et al. Damage-mediated phosphorylation of human p53 threonine 18 through a cascade mediated by a casein 1-like kinase. Effect on Mdm2 binding. J. Biol. Chem. 275, 9278–9283 (2000).
Chehab, N. H., Malikzay, A., Stavridi, E. S. & Halazonetis, T. D. Phosphorylation of serine 20 mediates stabilisation of human p53 in response to DNA damage. Proc. Natl Acad. Sci. USA 96, 13777–13782 (1999).
Hirao, A. et al. DNA damage-induced activation of p53 by the checkpoint kinase chk2. Science 287, 1824–1827 (2000).
Shieh, S.Y., Taya, Y. & Prives, C. DNA damage-inducible phosphorylation of p53 at N-terminal sites including a novel site, Ser20, requires tetramerisation. EMBO J. 18, 1815–1823 (1999).
Saito, S. et al. ATM mediates phosphorylation at multiple p53 sites, including Ser46, in response to ionizing radiation. J. Biol. Chem. 277, 12491–12494 (2002).
Saito, S. et al. Phosphorylation site interdependence of human p53 post-translational modifications in response to stress. J. Biol. Chem. 278, 37536–37544 (2003).
Sakaguchi, K. et al. DNA damage activates p53 through a phosphorylation-acetylation cascade. Genes Dev. 12, 2831–2841 (1998).
Dornan, D. & Hupp, T. R. Inhibition of p53-dependent transcription by BOX-I phospho-peptide mimetics that bind to p300. EMBO Rep. 2, 139–144 (2001).
Dumaz, N. & Meek, D. W. p53-serine15 phosphorylation stimulates transactivation function but does not directly influence interaction with HDM2. EMBO J. 18, 7002–7010 (1999).
Feng, H. et al. Structural basis for p300 Taz2–p53 TAD1 binding and modulation by phosphorylation. Structure 17, 202–210 (2009).
Jenkins, L. M. et al. Two distinct motifs within the p53 transactivation domain bind to the Taz2 domain of p300 and are differentially affected by phosphorylation. Biochemistry 48, 1244–1255 (2009).
Lambert, P. F., Kashanchi, F., Radonovich, M. F., Shiekhattar, R. & Brady, J. N. Phosphorylation of p53 serine 15 increases interaction with CBP. J. Biol. Chem. 273, 33048–33053 (1998).
Lee, C. W., Arai, M., Martinez-Yamout, M. A., Dyson, H. J. & Wright, P. E. Mapping the interactions of the p53 transactivation domain with the KIX domain of CBP. Biochemistry 48, 2115–2124 (2009).
Polley, S. et al. Differential recognition of phosphorylated transactivation domains of p53 by different p300 domains. J. Mol. Biol. 376, 8–12 (2008).
Teufel, D. P., Bycroft, M. & Fersht, A. R. Regulation by phosphorylation of the relative affinities of the N-terminal transactivation domains of p53 for p300 domains and Mdm2. Oncogene 28, 2112–2118 (2009).
Ito, A. et al. MDM2-HDAC1-mediated deacetylation of p53 is required for its degradation. EMBO J. 21, 6236–6245 (2002).
Craig, A. L. et al. Novel phosphorylation sites of human tumour suppressor protein p53 at ser20 and thr18 that disrupt the binding of mdm2 (mouse double minute 2) protein are modified in human cancers. Biochem. J. 342, 133–141 (1999).
Dumaz, N., Milne, D. M., Jardine, L. J. & Meek, D. W. Critical roles for the serine 20, but not the serine 15, phosphorylation site and for the polyproline domain in regulating p53 turnover. Biochem. J. 359, 459–464 (2001).
Jabbur, J. R. et al. Mdm-2 binding and TAFII31 recruitment is regulated by hydrogen bond disruption between the p53 residues Thr18 and Asp21. Oncogene 21, 7100–7113 (2002).
Unger, T. et al. Critical role for ser20 of human p53 in the negative regulation of p53 by MDM2. EMBO J. 18, 1805–1814 (1999).
Blattner, C., Hay, T. J., Meek, D. W. & Lane, D. P. Hypophosphorylation of Mdm2 augments p53 stability. Mol. Cell. Biol. 22, 6170–6182 (2002).
Boehme, K. A., Kulikov, R. & Blattner, C. p53 stabilization in response to DNA damage requires Akt/PKB and DNA-PK. Proc. Natl Acad. Sci. USA 105, 7785–90 (2008).
Bothner, B. et al. Defining the molecular basis of Arf and Hdm2 interactions. J. Mol. Biol. 314, 263–277 (2001).
Weber, J. D. et al. Cooperative signals governing ARF–mdm2 interaction and nucleolar localization of the complex. Mol. Cell. Biol. 20, 2517–2528 (2000).
Acknowledgements
My apology to the authors of many excellent studies which, owing to space limitations, I have been unable to explore and cite. I am grateful to Frances Fuller-Pace for critically reviewing the manuscript.
Author information
Authors and Affiliations
Related links
Related links
DATABASES
OMIM
FURTHER INFORMATION
Glossary
- Innate tumour suppression
-
Cellular mechanisms that detect and eliminate incipient tumour cells.
- Senescence
-
The irreversible withdrawal of cells from the proliferative cycle into a terminally non-proliferative state. When this state is promoted by oncogenes, it is often termed oncogene-induced senescence.
- Checkpoint pathway
-
A signal transduction pathway that is activated by stresses such as DNA damage, leading to the halting of a crucial biological process, such as DNA replication or cell division.
- Ataxia–telangiectasia
-
An inherited disease in which the absence of a functional ATM protein kinase gives rise to many disabilities, including a substantially increased risk of developing cancer.
- Focus
-
A sub-nuclear location at which DNA damage has occurred and to which DNA damage-associated proteins are specifically recruited.
- Fragile site
-
A chromosomal region that is highly susceptible to double-strand breaks under conditions of replication stress.
- Non-homologous end joining
-
A method of DNA repair in which the ends arising from a double-stranded break are recognized by specialized proteins and religated.
- Focus assay
-
A cell culture-based measurement of the neoplastic transformation of cells with respect to their ability to overcome contact inhibition.
Rights and permissions
About this article
Cite this article
Meek, D. Tumour suppression by p53: a role for the DNA damage response?. Nat Rev Cancer 9, 714–723 (2009). https://doi.org/10.1038/nrc2716
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrc2716
This article is cited by
-
Role of sestrins in metabolic and aging-related diseases
Biogerontology (2024)
-
A dual role of RBM42 in modulating splicing and translation of CDKN1A/p21 during DNA damage response
Nature Communications (2023)
-
Differential effects of pesticides on dioxin receptor signaling and p53 activation
Scientific Reports (2023)
-
Deer antlers: the fastest growing tissue with least cancer occurrence
Cell Death & Differentiation (2023)
-
CSE1L is a negative regulator of the RB-DREAM pathway in p53 wild-type NSCLC and can be targeted using an HDAC1/2 inhibitor
Scientific Reports (2023)