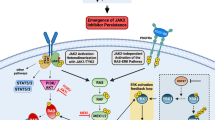
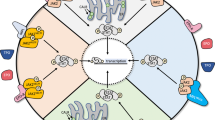
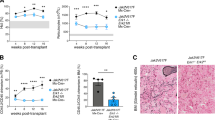
Key Points
-
Acquisition of the constitutively active Janus kinase 2 (JAK2)V617F mutation occurs in almost all patients with polycythaemia vera (PV) and in a significant number of patients with essential thombocythaemia (ET) and primary myelofibrosis (PMF).
-
JAK2V617F is a constitutively active tyrosine kinase that activates signal transducer and activator of transcription (Stat), mitogen activated protein kinase (MAPK) and phosphotidylinositol 3-kinase (PI3K) signalling pathways, and transforms haematopoietic progenitors.
-
The identification of JAK2V617F has had a significant impact on the classification, diagnosis and prognosis of PV, ET and PMF.
-
Gain-of-function mutations in JAK2 exon 12 and in the thrombopoietin receptor are observed in some patients with JAK2V617F-negative myeloproliferative disorders (MPD), suggesting constitutive activation of JAK2 signalling is central to the pathogenesis of PV, ET and PMF.
-
The presence of a single disease allele in related, but clinically distinct MPD indicates that additional genetic events contribute to the pathogenesis of these disorders.
-
The discovery of JAK2V617F has led to the development of selective JAK2 inhibitors for the treatment of PV, ET and PMF.
Abstract
The myeloproliferative disorders polycythaemia vera (PV), essential thombocythaemia (ET), and primary myelofibrosis (PMF) are clonal disorders of multipotent haematopoietic progenitors. The genetic cause of these diseases was not known until 2005, when several independent groups demonstrated that most patients with PV, ET and PMF acquire a single point mutation in the cytoplasmic tyrosine kinase JAK2 (JAK2V617F). These discoveries have changed the landscape for diagnosis and classification of PV, ET and PMF, and show the ability of genomic technologies to identify new molecular targets in human malignancies with pathogenetic, diagnostic and therapeutic significance.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Dameshek, W. Some speculations on the myeloproliferative syndromes. Blood 6, 372–375 (1951).
Bartram, C. R. et al. Translocation of c-ab1 oncogene correlates with the presence of a Philadelphia chromosome in chronic myelocytic leukaemia. Nature 306, 277–280 (1983).
Golub, T. R. et al. Fusion of PDGF receptor β to a novel ets-like gene, tel, in chronic myelomonocytic leukemia with t(5;12) chromosomal translocation. Cell 77, 307–316 (1994).
Cools, J. et al. A tyrosine kinase created by fusion of the PDGFRA and FIP1L1 genes as a therapeutic target of imatinib in idiopathic hypereosinophilic syndrome. N. Engl. J. Med. 348, 1201–1214 (2003).
Longley, B. J. et al. Somatic c-KIT activating mutation in urticaria pigmentosa and aggressive mastocytosis: establishment of clonality in a human mast cell neoplasm. Nature Genet. 12, 312–314. (1996).
Druker, B. J. et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N. Engl. J. Med. 344, 1031–1037 (2001).
Apperley, J. F. et al. Response to imatinib mesylate in patients with chronic myeloproliferative diseases with rearrangements of the platelet-derived growth factor receptor β. N. Engl. J. Med. 347, 481–487 (2002).
Levine, R. L. et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell 7, 387–397 (2005).
James, C. et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature 434, 1144–1148 (2005).
Baxter, E. J. et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet 365, 1054–1061 (2005).
Kralovics, R. et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N. Engl. J. Med. 352, 1779–1790 (2005). References 8–11 report the discovery of the JAK2V617F allele in PV, ET, and PMF.
Delhommeau, F. et al. Evidence that the JAK2 G1849T (V617F) mutation occurs in a lymphomyeloid progenitor in polycythemia vera and idiopathic myelofibrosis. Blood 109, 71–77 (2007).
Ishii, T. et al. Involvement of various hematopoietic cell lineages by the JAK2V617F mutation in polycythemia vera. Blood 108, 3128–3134 (2006).
Jamieson, C. H. et al. The JAK2 V617F mutation occurs in hematopoietic stem cells in polycythemia vera and predisposes toward erythroid differentiation. Proc. Natl Acad. Sci. USA 103, 6224–6229 (2006).
Huntly, B. J. et al. MOZ-TIF2, but not BCR-ABL, confers properties of leukemic stem cells to committed murine hematopoietic progenitors. Cancer Cell 6, 587–596 (2004).
Kralovics, R., Y. Guan & J. T. Prchal . Acquired uniparental disomy of chromosome 9p is a frequent stem cell defect in polycythemia vera. Exp. Hematol. 30, 229–236 (2002). This report identified acquired uniparental disomy of chromosome 9p24 in PV.
Scott, L. M. et al. Progenitors homozygous for the V617F JAK2 mutation occur in most patiens with polcythemia vera, but not essential thrombocythemia. Blood 108, 2435–2437 (2006).
Levine, R. L. et al. The JAK2V617F activating mutation occurs in chronic myelomonocytic leukemia and acute myeloid leukemia, but not in acute lymphoblastic leukemia or chronic lymphocytic leukemia. Blood 106, 3377–3379 (2005).
Jelinek, J. et al. JAK2 mutation 1849G >T is rare in acute leukemias but can be found in CMML, Philadelphia-chromosome negative CML and megakaryocytic leukemia. Blood 106, 3370–3373 (2005).
Levine, R. L. et al. X-inactivation based clonality analysis and quantitative JAK2V617F assessment reveals a strong association between clonality and JAK2V617F in PV but not ET/MMM, and identifies a subset of JAK2V617F negative ET and MMM patients with clonal hematopoiesis. Blood 107, 4039–4041 (2006).
Steensma, D. P. et al. The JAK2 V617F activating tyrosine kinase mutation is an infrequent event in both “atypical” myeloproliferative disorders and the myelodysplastic syndrome. Blood 106, 1207–1209 (2005).
Scott, L. M. et al. The V617F JAK2 mutation is uncommon in cancers and in myeloid malignancies other than the classic myeloproliferative disorders. Blood 106, 2920–2921 (2005).
Verma, A. et al. Jak family of kinases in cancer. Cancer Metastasis Rev. 22, 423–434 (2003).
Walters, D. K. et al. Activating alleles of JAK3 in acute megakaryoblastic leukemia. Cancer Cell 10, 65–75 (2006).
Joos, S. et al. Genomic imbalances including amplification of the tyrosine kinase gene JAK2 in CD30+ Hodgkin cells. Cancer Res. 60, 549–552 (2000).
Melzner, I. et al. Biallelic mutation of SOCS-1 impairs JAK2 degradation and sustains phospho-JAK2 action in the MedB-1 mediastinal lymphoma line. Blood 105, 2535–2542 (2005).
Weniger, M. A. et al. Mutations of the tumor suppressor gene SOCS-1 in classical Hodgkin lymphoma are frequent and associated with nuclear phospho-STAT5 accumulation. Oncogene 25, 2679–2684 (2006).
Melzner, I. et al. Biallelic deletion within 16p13.13 including SOCS-1 in Karpas1106P mediastinal B-cell lymphoma line is associated with delayed degradation of JAK2 protein. Int. J. Cancer 118, 1941–1944 (2006).
Galm, O. et al. SOCS-1, a negative regulator of cytokine signaling, is frequently silenced by methylation in multiple myeloma. Blood 101, 2784–2788 (2003).
Parganas, E. et al. Jak2 is essential for signaling through a variety of cytokine receptors. Cell 93, 385–395 (1998).
Saharinen, P., K. Takaluoma & O. Silvennoinen . Regulation of the Jak2 tyrosine kinase by its pseudokinase domain. Mol. Cell Biol. 20, 3387–3395 (2000).
Lindauer, K. et al. Prediction of the structure of human Janus kinase 2 (JAK2) comprising the two carboxy-terminal domains reveals a mechanism for autoregulation. Protein Eng. 14, 27–37 (2001).
Ihle, J. N. & Gilliland, D. G. Jak2: normal function and role in hematopoietic disorders. Curr. Opin. Genet. Dev. 17, 8–14 (2007).
Zhao, R. et al. Identification of an acquired JAK2 mutation in polycythemia vera. J. Biol. Chem. 280, 22788–22792 (2005).
Prchal, J. F. & Axelrad, A. A. Bone-marrow responses in polycythemia vera. N. Engl. J. Med. 290, 1382 (1974).
Lu, X. et al. Expression of a homodimeric type I cytokine receptor is required for JAK2V617F-mediated transformation. Proc. Natl Acad. Sci. USA 102, 18962–18967 (2005).
Garcon, L. et al. Constitutive activation of STAT5 and Bcl-xL overexpression can induce endogenous erythroid colony formation in human primary cells. Blood 108, 1551–1554 (2006).
Roder, S. et al. STAT3 is constitutively active in some patients with Polycythemia rubra vera. Exp. Hematol. 29, 694–702 (2001).
Silva, M. et al. Expression of Bcl-x in erythroid precursors from patients with polycythemia vera. N. Engl. J. Med. 338, 564–571 (1998).
Schwaller, J. et al. Stat5 is essential for the myelo- and lymphoproliferative disease induced by TEL/JAK2. Mol. Cell. 6, 693–704 (2000).
Nicholson, S. E. et al. Mutational analyses of the SOCS proteins suggest a dual domain requirement but distinct mechanisms for inhibition of LIF and IL-6 signal transduction. EMBO J. 18, 375–385 (1999).
Sasaki, A. et al. CIS3/SOCS-3 suppresses erythropoietin (EPO) signaling by binding the EPO receptor and JAK2. J. Biol. Chem. 275, 29338–29347 (2000).
Frantsve, J. et al. Socs-1 inhibits TEL-JAK2-mediated transformation of hematopoietic cells through inhibition of JAK2 kinase activity and induction of proteasome-mediated degradation. Mol. Cell Biol. 21, 3547–3557 (2001).
Hookham, M. B. et al. The myeloproliferative disorder-associated JAK2 V617F mutant escapes negative regulation by suppressor of cytokine signaling 3. Blood 109, 4924–4929. (2007).
Wernig, G. et al. Expression of Jak2V617F causes a polycythemia vera-like disease with associated myelofibrosis in a murine bone marrow transplant model. Blood 107, 4274–4281 (2006).
Lacout, C. et al. JAK2V617F expression in murine hematopoietic cells leads to MPD mimicking human PV with secondary myelofibrosis. Blood 108, 1652–1660 (2006).
Zaleskas, V. M. et al. Molecular pathogenesis and therapy of polycythemia induced in mice by JAK2 V617F. PLoS ONE 1e18, (2006).
Bumm, T. G. et al. Characterization of murine JAK2V617F-positive myeloproliferative disease. Cancer Res. 66, 11156–11165 (2006). References 45–48 report that expression of JAK2V617F in a murine bone marrow transplant model results in a PV phenotype.
Daley, G., Van Etten, R. & Baltimore, D. Induction of chronic myelogenous leukemia in mice by the p210bcr/abl gene of the Philadelphia chromosome. Science 247, 824–830 (1990).
Cools, J. et al. PKC412 overcomes resistance to imatinib in a murine model of FIP1L1-PDGFRα-induced myeloproliferative disease. Cancer Cell 3, 459–469 (2003).
Campbell, P. J. et al. Mutation of JAK2 in the myeloproliferative disorders: timing, clonality studies, cytogenetic associations, and role in leukemic transformation. Blood 108, 3548–3555 (2006).
Jones, A. V. et al. Widespread occurrence of the JAK2 V617F mutation in chronic myeloproliferative disorders. Blood 106, 2162–2168 (2005).
Scott, L. M. et al. JAK2 exon 12 mutations in polycythemia vera and idiopathic erythrocytosis. N. Engl. J. Med. 356, 459–468 (2007). This paper identifies somatic mutations in JAK2 exon 12 in JAK2V617F -negative PV.
Arcasoy, M. O. et al. Familial erythrocytosis associated with a short deletion in the erythropoietin receptor gene. Blood 89, 4628–4635 (1997).
Pikman, Y. et al. MPLW515L is a novel somatic activating mutation in myelofibrosis with myeloid metaplasia PLoS Med. 3, e270 (2006). This paper reports gain-of-function mutations in the thrombopoietin receptor in JAK2V617F -negative PMF.
Ding, J. et al. Familial essential thrombocythemia associated with a dominant-positive activating mutation of the c-MPL gene, which encodes for the receptor for thrombopoietin. Blood 103, 4198–4200 (2004).
Pardanani, A. D. et al. MPL515 mutations in myeloproliferative and other myeloid disorders: a study of 1182 patients. Blood 108, 3472–3476 (2006).
Roberts, A. E. et al. Germline gain-of-function mutations in SOS1 cause Noonan syndrome. Nature Genet. 39, 70–74 (2007).
Tartaglia, M. et al. Gain-of-function SOS1 mutations cause a distinctive form of Noonan syndrome. Nature Genet. 39, 75–79 (2007).
Gilbert, H. S. Familial myeloproliferative disease. Baillieres Clin. Haematol. 11, 849–858 (1998).
Skoda, R. & Prchal, J. T. Lessons from familial myeloproliferative disorders. Semin. Hematol. 42, 266–273 (2005).
Schubbert, S. et al. Germline KRAS mutations cause Noonan syndrome. Nature Genet. 38, 331–336 (2006).
Mulligan, L. M. et al. Germ-line mutations of the RET proto-oncogene in multiple endocrine neoplasia type 2A. Nature 363, 458–460 (1993).
Cario, H. et al. The JAK2V617F mutation is acquired secondary to the predisposing alteration in familial polycythaemia vera. Br. J. Haematol. 130, 800–801 (2005).
Bellanne-Chantelot, C. et al. Genetic and clinical implications of the Val617Phe JAK2 mutation in 72 families with myeloproliferative disorders. Blood 108 346–352 (2006).
Theocharides, A. et al. Leukemic blasts in transformed JAK2-V617F positive myeloproliferative disorders are frequently negative for the JAK2-V617F mutation. Blood 110, 375–379 (2007).
Asimakopoulos, F. A. et al. Molecular analysis of chromosome 20q deletions associated with myeloproliferative disorders and myelodyslastic syndromes. Blood 84, 3086–3094 (1994).
Kralovics, R. et al. Acquisition of the V617F mutation of JAK2 is a late genetic event in a subset of patients with myeloproliferative disorders. Blood 108, 1377–1380 (2006).
Murphy, S. et al. Experience of the Polycythemia Vera Study Group with essential thrombocythemia: a final report on diagnostic criteria, survival, and leukemic transition by treatment. Semin. Hematol. 34, 29–39 (1997).
Campbell, P. J. et al. Definition of subtypes of essential thrombocythaemia and relation to polycythaemia vera based on JAK2 V617F mutation status: a prospective study. Lancet 366, 1945–1953 (2005).
Tefferi, A. et al. Proposals and rationale for revision of the World Health Organization diagnostic criteria for polycythemia vera, essential thrombocythemia, and primary myelofibrosis: recommendations from an ad hoc international expert panel. Blood, 8 May 2007 (doi 10.1182/blood-2007-04-083501).
Tefferi, A. & Pardanani, A. Evaluation of increased hemoglobin in the JAK2 mutations era. Mayo Clin. Proc. 82, 559–606 (2007).
Tefferi, A. & Vardiman, J. W. The diagnostic interface between histology and molecular tests in myeloproliferative disorders. Curr. Opin. Hematol. 14, 115–122 (2007).
Thiele, J. & Kvasnicka, H. M. Hematopathologic findings in chronic idiopathic myelofibrosis. Semin. Oncol. 32, 380–394 (2005).
Tefferi, A. et al. Bone marrow JAK2V617F allele burden and clinical correlates in polycythemia vera. Leukemia 28 June 2007 (doi: 10.1038/sj.leu.2404810).
Vannucchi, A. M. et al. Influence of the Jak2V617F mutational load at diagnosis on major clinical aspects in patients with polycythemia vera. Blood 108, Abst. 5 (2006)
Finazzi, G. et al. Risk of thrombosis in patients with essential thrombocythemia and polycythemia vera according to JAK2 V617F mutation status. Haematologica 92, 135–136 (2007).
Antonioli, E. et al. Clinical implications of the JAK2 V617F mutation in essential thrombocythemia. Leukemia 19, 1847–1849 (2005).
Wolanskyj, A. P. et al. JAK2 mutation in essential thrombocythaemia: clinical associations and long-term prognostic relevance. Br. J. Haematol. 131, 208–213 (2005).
Tefferi, A. et al. The JAK2(V617F) tyrosine kinase mutation in myelofibrosis with myeloid metaplasia: lineage specificity and clinical correlates. Br. J. Haematol. 131, 320–328 (2005).
Campbell, P. J. et al. V617F mutation in JAK2 is associated with poorer survival in idiopathic myelofibrosis. Blood 107, 2098–2100 (2006).
Mesa, R. A. et al. JAK2(V617F) and leukemic transformation in myelofibrosis with myeloid metaplasia. Leuk. Res. 30, 1457–1460 (2006).
Deeg, H. J. et al. Allogeneic hematopoietic stem cell transplantation for myelofibrosis. Blood 102, 3912–3918 (2003).
Guardiola, P. et al. Allogeneic stem cell transplantation for agnogenic myeloid metaplasia: a European Group for Blood and Marrow Transplantation, Societe Francaise de Greffe de Moelle, Gruppo Italiano per il Trapianto del Midollo Osseo, and Fred Hutchinson Cancer Research Center Collaborative Study. Blood 93, 2831–2838 (1999).
Kroger, N. et al. Pilot study of reduced-intensity conditioning followed by allogeneic stem cell transplantation from related and unrelated donors in patients with myelofibrosis. Br. J. Haematol. 128, 690–697 (2005).
Rondelli, D. et al. Allogeneic hematopoietic stem-cell transplantation with reduced-intensity conditioning in intermediate- or high-risk patients with myelofibrosis with myeloid metaplasia. Blood 105, 4115–4119 (2005).
Druker, B. et al. Efficacy and safety of a specific inhibitor of the Bcr-Abl tyrosine kinase in chronic myeloid leukaemia. N. Engl. J. Med. 344, 1031–1037 (2001).
Pardanani, A. et al. TG101209, a small molecule JAK2-selective kinase inhibitor potently inhibits myeloproliferative disorder-associated JAK2V617F and MPLW515L/K mutations. Leukemia, 21, 1658–1668 (2007). This paper describes the development of a rationally designed inhibitor of JAK2 which potently inhibits the growth of JAK2V617F- and MPLW515L-positive haematopoietic progenitors.
Dobrzanski, P. et al. CEP-701 Is a JAK2 inhibitor which attenuates JAK2/STAT5 signaling pathway and the proliferation of primary cells from patients with myeloproliferative disorders. Blood 108, abst. 3594 (2006).
Geron, I. et al. Inhibition of JAK2 V617F-induced erythroid skewing of hematopoietic stem cell differentiation with a selective JAK2 antagonist. Blood 108, abst. 3616 (2006).
Gourley, E. S. et al. Discovery and characterizatio of small molecule inhibitors of JAK2. American Association for Cancer Research Proceedings, abst. 2387, 2007.
Russell, S. M. et al. Mutation of Jak3 in a patient with SCID: essential role of Jak3 in lymphoid development. Science 270, 797–800 (1995).
Macchi, P. et al. Mutations of Jak-3 gene in patients with autosomal severe combined immune deficiency (SCID). Nature 377, 65–68 (1995).
Samuelsson, J. et al. Limited effects on JAK2 mutational status after pegylated interferon a-2b therapy in polycythemia vera and essential thrombocythemia. Haematologica 91, 1281–1282 (2006).
Kiladjian, J. J. et al. High molecular response rate of polycythemia vera patients treated with pegylated interferon α-2a. Blood 108, 2037–2040 (2006).
Jones, A. V. et al. Minimal molecular response in polycythemia vera patients treated with imatinib or interferon α. Blood 107, 3339–3341 (2006).
Ruiz-Arguelles, G. J. et al. Clearance of the Janus kinase 2 (JAK2) V617F mutation after allogeneic stem cell transplantation in a patient with myelofibrosis with myeloid metaplasia. Am. J. Hematol. 82, 400–402 (2006).
Tefferi, A. et al. Lenalidomide therapy in del(5)(q31)-associated myelofibrosis: cytogenetic and JAK2V617F molecular remissions. Leukemia 8, 1827–1828 (2007).
Gorre, M. E. et al. Clinical resistance to STI-571 cancer therapy caused by BCR-ABL gene mutation or amplification. Science 293, 876–880 (2001).
Shah, N. P. et al. Multiple BCR-ABL kinase domain mutations confer polyclonal resistance to the tyrosine kinase inhibitor imatinib (STI571) in chronic phase and blast crisis chronic myeloid leukemia. Cancer Cell 2, 117–125 (2002).
Azam, M., Latek, R. R. & Daley, G. Q. Mechanisms of autoinhibition and STI-571/imatinib resistance revealed by mutagenesis of BCR-ABL. Cell 112, 831–843 (2003).
Shah, N. P. et al. Overriding imatinib resistance with a novel ABL kinase inhibitor. Science 305, 399–401 (2004).
Talpaz, M. et al. Dasatinib in imatinib-resistant Philadelphia chromosome-positive leukemias. N. Engl. J. Med. 354, 2531–2541 (2006).
Kantarjian, H. et al. Nilotinib in imatinib-resistant CML and Philadelphia chromosome-positive ALL. N. Engl. J. Med. 354, 2542–2551 (2006).
Weisberg, E. et al. Characterization of AMN107, a selective inhibitor of wild-type and mutant Bcr-Abl. Cancer Cell 7, 129–141 (2005).
Mercher, T. et al. JAK2T875N is a novel activating mutation that results in myeloproliferative disease with features of megakaryoblastic leukemia in a murine bone marrow transplantation model. Blood 108, 2770–2779 (2006).
Malinge, S. et al. Novel activating JAK2 mutation in a patient with Down syndrome and B-cell precursor acute lymphoblastic leukemia. Blood 109, 2202–2204 (2007).
Landolfi, R. et al., Leukocytosis as a major thrombotic risk factor in patients with polycythemia vera. Blood 109, 2446–2452 (2007).
Carobbio, A., et al. Leukocytosis is a risk factor for thrombosis in essential thrombocythemia: interaction with treatment, standard risk factors, and Jak2 mutation status. Blood 109, 2310–2313 (2007).
Falanga, A., et al. V617F JAK-2 mutation in patients with essential thrombocythemia: relation to platelet, granulocyte, and plasma hemostatic and inflammatory molecules. Exp. Hematol. 35, 702–711 (2007).
Gilliland, D. G. et al. Clonality in myeloproliferative disorders: analysis by means of the polymerase chain reaction. Proc. Natl Acad. Sci. USA 88, 6848–6852 (1991).
Adamson, J. W. et al. Polycythemia vera: stem-cell and probable clonal origin of the disease. N. Engl. J. Med. 295, 913–916 (1976).
Elliott, M. A. & Tefferi, A. Thrombosis and haemorrhage in polycythaemia vera and essential thrombocythaemia. Br. J. Haematol. 128, 275–290 (2005).
Tefferi, A. et al. Validation and comparison of contemporary prognostic models in primary myelofibrosis: analysis based on 334 patients from a single institution. Cancer 109, 2083–2088 (2007).
Acknowledgements
The Gililand Laboratory is supported by the US National Institutes of Health, the Howard Hughes Medical institute, the Leukemia and Lymphoma Society, and the Doris Duke Charitable Foundation. The Gilliland and Tefferi Laboratories are supported in part by a grant from the Myeloproliferative Disorders Foundation. D.G.G. is an Investigator of the Howard Hughes Medical Institute, and a Doris Duke Charitable Foundation Distinguished Clinical Scientist Award recipient. R.L.L. is an American Society of Hematology Basic Research Fellow Award recipient, and a Doris Duke Charitable Foundation Clinical Scientist Development Award recipient.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- Polycythaemia vera
-
(PV). Chronic blood disorder characterized by increased red blood cell count.
- Thrombocythaemia
-
Increased platelet count.
- Mastocytosis
-
Chronic blood disorder characterized by an increased number of mast cells.
- Uniparental disomy
-
Homozygosity at a genetic locus caused by mitotic recombination and duplication of either the maternal or paternal allele.
- Cytokine receptor scaffold
-
Intracellular component of cytokine receptors that engages Janus kinases and enables the activation of signal transduction.
- Leucopaenia
-
Low white blood cell count.
- Erythrocytosis
-
Increased red blood cell count.
- Leukocytosis
-
Increased white blood cell count.
- Forme fruste
-
An atypical, especially a mild or incomplete, form of a disease.
- Haematocrit
-
Increase in the cellular fraction of peripheral blood, caused by an increased red blood cell count.
- Reduced intensity conditioning
-
Bone marrow transplantation strategy using lower doses of chemotherapy and radiation to minimize toxicity.
- Anagrelide
-
A drug used to decrease the number of platelets in the blood of patients who have a myeloproliferative disorder.
Rights and permissions
About this article
Cite this article
Levine, R., Pardanani, A., Tefferi, A. et al. Role of JAK2 in the pathogenesis and therapy of myeloproliferative disorders. Nat Rev Cancer 7, 673–683 (2007). https://doi.org/10.1038/nrc2210
Issue Date:
DOI: https://doi.org/10.1038/nrc2210
This article is cited by
-
Gut-Brain Axis Modulation of Metabolic Disorders: Exploring the Intertwined Neurohumoral Pathways and Therapeutic Prospects
Neurochemical Research (2024)
-
The therapeutic potential of neurofibromin signaling pathways and binding partners
Communications Biology (2023)
-
Essential Thrombocythemia in Adolescents and Young Adults: Clinical Aspects, Treatment Options and Unmet Medical Needs
Current Treatment Options in Oncology (2023)
-
Enhanced clinical assessment of hematologic malignancies through routine paired tumor and normal sequencing
Nature Communications (2023)
-
JAK2 inhibitor persistence in MPN: uncovering a central role of ERK activation
Blood Cancer Journal (2022)