Key Points
-
Anti-mitotic therapies that target tubulin are effective and widely used in treating cancer, but they have limitations related to the role of tubulin in the cytoskeleton of normal cells.
-
New compounds that inhibit new targets with specific functions in mitosis have now been identified, and show promising anti-tumour activity in preclinical model systems.
-
Early clinical studies have begun to show the pharmacodynamic activities of these new compounds in cancer patients.
-
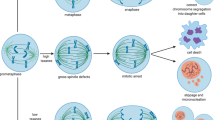
These new mitotic inhibitors are very effective at preventing the proliferation of most tumour cells in vitro, but the subsequent cellular response to cell-cycle arrest is quite varied and includes apoptosis, mitotic catastrophe, mitotic slippage, senescence and reversible mitotic arrest depending on what cell line and/or inhibitor is studied.
-
At present, the genetic or biochemical factors that define how a particular tumour cell will respond to mitotic injuries are poorly understood, but will be very important in helping to identify which patients will be the best candidates for treatment with these new agents.
Abstract
The advent of molecularly targeted drug discovery has facilitated the identification of a new generation of anti-mitotic therapies that target proteins with specific functions in mitosis. The exquisite selectivity for mitosis and the distinct ways in which these new agents interfere with mitosis provides the potential to not only overcome certain limitations of current tubulin-targeted anti-mitotic drugs, but to expand the scope of clinical efficacy that those drugs have established. The development of these new anti-mitotic drugs as targeted therapies faces significant challenges; nevertheless, these potential therapies also serve as unique tools to dissect the molecular mechanisms of the mitotic-checkpoint response.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Wood, K. W. et al. Past and future of the mitotic spindle as an oncology target. Curr. Opin. Pharmacol. 1, 370–377 (2001).
Nigg, E. A., Blangy, A. & Lane, H. A. Dynamic changes in nuclear architecture during mitosis: on the role of protein phosphorylation in spindle assembly and chromosome segregation. Exp. Cell Res. 229, 174–180 (1996).
Keen, N. & Taylor, S. Aurora-kinase inhibitors as anticancer agents. Nature Rev. Cancer 4, 927–936 (2004).
Strebhardt, K., Ullrich, A., Authors, F. N. & Ullrich, A. Targeting polo-like kinase 1 for cancer therapy. Nature Rev. Cancer 6, 321–330 (2006).
Blangy, A. et al. Phosphorylation by p34cdc2 regulates spindle association of human Eg5,a kinesin-related motor essential for bipolar spindle formation in vivo. Cell 83, 1159–1169 (1995).
Schaar, B. T. et al. CENP-E function at kinetochores is essential for chromosome alignment. J. Cell Biol. 139, 1373–1382 (1997).
Wood, K. W. et al. CENP-E is a plus end-directed kinetochore motor required for metaphase chromosome alignment. Cell 91, 357–366 (1997).
Abrieu, A. et al. CENP-E as an essential component of the mitotic checkpoint in vitro. Cell 102, 817–826 (2000). This thought-provoking study shows the crucial role of CENPE in mitotic-spindle checkpoint regulation in addition to its previously known role in chromosome alignment.
Tao, W. et al. Induction of apoptosis by an inhibitor of the mitotic kinesin KSP requires both activation of the spindle assembly checkpoint and mitotic slippage. Cancer Cell 8, 49–59 (2005). This study shows that mitotic arrest alone can be insufficient to cause tumour cell death, and that subsequent events ultimately define cell fate.
Jordan, M. A. et al. Mitotic block induced in HeLa cells by low concentrations of paclitaxel (Taxol) results in abnormal mitotic exit and apoptotic cell death. Cancer Res. 56, 816–825 (1996).
Marcus, A. I. et al. Mitotic kinesin inhibitors induce mitotic arrest and cell death in Taxol-resistant and-sensitive cancer cells. J. Biol. Chem. 280, 11569–11577 (2005).
Castedo, M. et al. Cell death by mitotic catastrophe: a molecular definition. Oncogene 23, 2825–2837 (2004).
Michel, L. et al. Complete loss of the tumor suppressor MAD2 causes premature cyclin B degradation and mitotic failure in human somatic cells. Proc. Natl Acad. Sci. USA 101, 4459–4464 (2004).
Kops, G. J. P. L., Foltz, D. R. & Cleveland, D. W. Lethality to human cancer cells through massive chromosome loss by inhibition of the mitotic checkpoint. Proc. Natl Acad. Sci. USA 101, 8699–8704 (2004).
Michel, L., Benezra, R. & Diaz-Rodriguez, E. A double edged sword: MAD2 dependent mitotic checkpoint defects in tumorigenesis and tumor cell death. Cell Cycle 3, 990–992 (2006).
Ditchfield, C. et al. Aurora B couples chromosome alignment with anaphase by targeting BubR1, Mad2, and Cenp-E to kinetochores. J. Cell Biol. 161, 267–280 (2003).
Kashina, A. S. et al. The bimC family of kinesins: essential bipolar mitotic motors driving centrosome separation. Biochimica Biophysica Acta 1357, 257–271 (1997).
Hegde, P. S. et al. Differential gene expression analysis of kinesin spindle protein in human solid tumors. Proc. Am. Soc. Clin. Oncol. 22, 535 (2003).
Mayer, T. U. et al. Small molecule inhibitor of mitotic spindle bipolarity identified in a phenotype-based screen. Science 286, 971–974 (1999). The first study to show that selective inhibitors of KSP could be identified and shown to have excellent anti-mitotic activity
Sakowicz, R. et al. Antitumor activity of a kinesin inhibitor. Cancer Res. 64, 3276–3280 (2004).
Brier, S. et al. Identification of the protein binding region of S-trityl-L-cysteine, a new potent inhibitor of the mitotic kinesin Eg5. Biochemistry 43, 13072–13082 (2004).
Debonis, S. et al. In vitro screening for inhibitors of the human mitotic kinesin Eg5 with antimitotic and antitumor activities. Mol. Cancer Ther. 3, 1079–1090 (2004).
Gartner, M. et al. Development and biological evaluation of potent and specific inhibitors of mitotic Kinesin Eg5. Chembiochem. 6, 1173–1177 (2005).
Sunder-Plassmann, N. et al. Synthesis and biological evaluation of new tetrahydro-beta-carbolines as inhibitors of the mitotic kinesin Eg5. Bioorg. Med. Chem. 13, 6094–6111 (2005).
Cox, C. D. et al. Kinesin spindle protein (KSP) inhibitors. Part 1: the discovery of 3, 5-diaryl-4, 5-dihydropyrazoles as potent and selective inhibitors of the mitotic kinesin KSP. Bioorg. Med. Chem. Lett. 15, 2041–2045 (2005).
Yan, Y. et al. Inhibition of a mitotic motor protein: where, how, and conformational consequences. J. Mol. Biol. 335, 547–554 (2004).
Johnson, R. K. et al. SB-715992, a potent and selective inhbitor of the mitotic kinesin KSP, demonstrates broad-spectrum activity in advanced murine tumors and human tumor xenografts. Proc. Am. Assoc. Can. Res. 43, 269 (2002).
Gonzales, P. et al. Breadth of anti-tumor activity of CK0238273 (SB-715992), a novel inhibitor of the mitotic kinesin KSP. Proc. Am. Assoc. Can. Res. 43, 269 (2002).
Lobell, R. B. et al. In vivo characterization of an inhibitor of the mitotic kinesin, KSP: pharacodynamics, efficacy, and tolerability in xenograft tumor models. Proc. AACR-NCI-EORTC Mol. Tar. Can. Ther. Mtg Abst. B 189 (2005).
Garcia-Saez, I. et al. Crystal structure of the motor domain of the human kinetochore protein CENP-E. J. Mol. Biol. 340, 1107–1116 (2004).
Liu, D. et al. Interaction of Skp1 with CENP-E at the midbody is essential for cytokinesis. Biochem. Biophys. Res. Comm. 345, 394–402 (2006).
Yao, X. et al. The microtubule-dependent motor centromere-associated protein E (CENP-E) is an integral component of kinetochore corona fibers that link centromeres to spindle microtubules. J. Cell Biol. 139, 435–447 (1997).
Chan, G. K. et al. Human BUBR1 is a mitotic checkpoint kinase that monitors CENP-E functions at kinetochores and binds the cyclosome/APC. J. Cell Biol. 146, 941–954 (1999).
Mao, Y. et al. Activating and silencing the mitotic checkpoint through CENP-E-dependent activation/inactivation of BubR1. Cell 114, 87–98 (2003).
McEwen, B. F. et al. CENP-E is essential for reliable bioriented spindle attachment, but chromosome alignment can be achieved via redundant mechanisms in mammalian cells. Mol. Biol. Cell 12, 2776–2789 (2001).
Yao, X. et al. CENP-E forms a link between attachment of spindle microtubules to kinetochores and the mitotic checkpoint. Nature Cell Biol. 2, 484–491 (2000).
Kapoor, T. M. et al. Chromosomes can congress to the metaphase plate before biorientation. Science 311, 388–391 (2006).
Putkey, F. R. et al. Unstable kinetochore-microtubule capture and chromosomal instability following deletion of CENP-E. Dev. Cell 3, 351–365 (2002).
Andrews, P. D., Knatko, E., Moore, W. J. & Swedlow, J. R. Mitotic mechanics: the auroras come into view. Curr. Opin. Cell Biol. 15, 672–683 (2003).
Marumoto, T., Zhang, D. & Saya, H. Aurora-A- a guardian of poles. Nature Rev. Cancer 5, 42–50 (2005).
Matthews, N., Visintin, C., Hartzoulakis, B., Jarvis, A. & Selwood, D. L. Aurora A and B kinases as targets for cancer: will they be selective for tumors? Expert Rev. Anticancer Ther. 6, 109–120 (2006).
Ecsedy, J. A. et al. Effect of aurora A inhibition in cultured human tumor cells using the selective small molecule inhibitor MLN8054. AACR Meeting Abstracts 2006 488 (2006).
Giet, R. et al. Drosophila Aurora A kinase is required to localize D-TACC to centrosomes and to regulate astral microtubules. J. Cell Biol. 156, 437–451 (2002).
Hannak, E., Kirkham, M., Hyman, A. A. & Oegema, K. Aurora-A kinase is required for centrosome maturation in Caenorhabditis elegans. J. Cell Biol. 155, 1109–1116 (2001).
Adams, R. R., Maiato, H., Earnshaw, W. C. & Carmena, M. Essential roles of Drosophila inner centromere protein (INCENP) and aurora B in histone H3 phosphorylation, metaphase chromosome alignment, kinetochore disjunction, and chromosome segregation. J. Cell Biol. 153, 865–880 (2001).
Hauf, S. et al. The small molecule Hesperadin reveals a role for Aurora B in correcting kinetochore-microtubule attachment and in maintaining the spindle assembly checkpoint. J. Cell Biol. 161, 281–294 (2003).
Gizatullin, F. et al. The Aurora kinase inhibitor VX-680 induces endoreduplication and apoptosis preferentially in cells with compromised p53-dependent postmitotic checkpoint function. Cancer Res. 66, 7668–7677 (2006).
Yang, H. et al. Mitotic requirement for aurora A kinase is bypassed in the absence of aurora B kinase. FEBS Lett. 579, 3385–3391 (2005).
Hoover, R. R. & Harding, M. W. Synergistic activity of the aurora kinase inhibitor MK-0457 (VX-680) with idarubicin, Ara-C, and inhibitors of BCR-ABL. ASH Annual Meeting Abstracts 108, 1384 (2006).
Barr, F. A., Sillje, H. H. W. & Nigg, E. A. Polo-like kinases and the orchestration of cell division. Nature Rev. Mol. Cell Biol. 5, 429–440 (2004).
Takai, N., Hamanaka, R., Yoshimatsu, J. & Miyakawa, I. Polo-like kinases (Plks) and cancer. Oncogene 24, 287–291 (2005).
Eckerdt, F., Yuan, J. & Strebhardt, K. Polo-like kinases and oncogenesis. Oncogene 24, 267–276 (2005).
Sumara, I. et al. Roles of polo-like kinase 1 in the assembly of functional mitotic spindles. Curr. Biol. 14, 1712–1722 (2004).
Liu, X. Q. & Erikson, R. L. Polo-like kinase (Plk)1 depletion induces apoptosis in cancer cells. Proc. Natl Acad. Sci. USA 100, 5789–5794 (2003).
Chu, Q. S. et al. Phase I trial of novel kinesin spindle protein (KSP) inhibitor SB-715992 IV Q 21 days. J. Clin. Oncol. 22, 2078 (2004).
Chu, Q. S. et al. A phase I study to determine the safety and pharmacokinetics of IV administered SB-715992, a novel kinesin spindle protein (KSP) inhibitor, in patients (pts) with solid tumors. Proc. Am. Soc. Clin. Oncol. 22, 525 (2003).
Burris, H. A. et al. Phase I trial of novel kinesin spindle protein (KSP) inhibitor SB-715992 IV days 1, 8, 15 q 28 days. J. Clin. Oncol. 22, 2004 (2004).
Miller, K. et al. Phase II, open label study of SB-715992 (ispinesib) in subjects with advanced or metastatic breast cancer. San Antonio Breast Cancer Symp. abst. 1089 (2005).
Jackson, J. R. et al. A second generation KSP inhibitor, SB-743921, is a highly potent and active therapeutic in preclinical models of cancer. AACR Meeting Abstracts 2006, B11 (2006).
Holen, K. D. et al. Phase I study to determine tolerability and pharmacokinetics (PK) of SB-743921, a novel kinesin spindle protein (KSP) inhibitor. J. Clin. Oncol. 24, 2000 (2006).
Holen, K. D. et al. Phase I study to determine tolerability and pharmacokinetics (PK) of SB-743921, a novel kinesin spindle protein (KSP) inhibitor. J. Clin. Oncol. 23, 2010 (2005).
Stein, M. N. et al. Phase I clinical and pharmacokinetic (PK) trial of the kinesin spindle protein (KSP) inhibitor MK-0731 in cancer patients. J. Clin. Oncol. 24, 2001 (2006).
Rubin, E. H. et al. A phase I clinical and pharmacokinetic (PK) trial of the aurora kinase (AK) inhibitor MK-0457 in cancer patients. J. Clin. Oncol. 24, 3009 (2006).
Schellens, J. H. et al. Phase I and pharmacological study of the novel aurora kinase inhibitor AZD1152. J. Clin. Oncol. 24, 3008 (2006).
Hofheinz, R. et al. A phase I repeated dose escalation study of the Polo-like kinase 1 inhibitor BI 2536 in patients with advanced solid tumours. J. Clin. Oncol. 24, 2038 (2006).
Munzert, G. et al. A phase I study of two administration schedules of the Polo-like kinase 1 inhibitor BI 2536 in patients with advanced solid tumors. J. Clin. Oncol. 24, 3069 (2006).
Ohnuma, T. et al. Phase I study of ON 01910. Na by 3-day continuous infusion (CI) in patients (pts) with advanced cancer. J. Clin. Oncol. 24, 13137 (2006).
Donehower, R. C. et al. Phase I study of ON-01910. Na, a novel cell cycle inhibitor in adult patients with solid tumors. J. Clin. Oncol. 24, 13026 (2006).
Steegmaier, M. et al. BI 2536, a potent and highly selective inhibitor of Polo-like kinase 1 (Plk1), induces mitotic arrest and apoptosis in a broad spectrum of tumor cell lines. Clin. Cancer Res. 11, 9147 (2005).
Cogswell, J. P., Brown, C. E., Bisi, J. E. & Neill, S. D. Dominant-negative polo-like kinase 1 induces mitotic catastrophe independent of cdc25C function. Cell Growth Differ. 11, 615–623 (2000).
Soncini, C. et al. PHA-680632, a novel aurora kinase inhibitor with potent antitumoral activity. Clin. Cancer Res. 12, 4080–4089 (2006).
Chang, J. C. et al. Gene expression profiling for the prediction of therapeutic response to docetaxel in patients with breast cancer. Lancet 362, 362–369 (2003). This landmark study showed that gene-expression profiles from patient tumour samples can predict whether the disease will respond to taxane therapy.
Gianni, L. et al. Gene expression profiles in paraffin-embedded core biopsy tissue predict response to chemotherapy in women with locally advanced breast cancer. J. Clin. Oncol. 23, 7265–7277 (2005).
Komatsu, M. et al. Prediction of individual response to platinum/paclitaxel combination using novel marker genes in ovarian cancers. Mol. Cancer Ther. 5, 767–775 (2006).
Magne, N., Largillier, R., Marcy, P. Y., Magne, J. & Namer, M. Cardiac toxicity assessment in locally advanced breast cancer treated neoadjuvantly with doxorubicin/paclitaxel regimen. Supp. Care Cancer 13, 819–825 (2005).
Holmes, F. A. et al. Sequence-dependent alteration of doxorubicin pharmacokinetics by paclitaxel in a phase i study of paclitaxel and doxorubicin in patients with metastatic breast cancer. J. Clin. Oncol. 14, 2713–2721 (1996).
Mondesire, W. H. et al. Targeting mammalian target of rapamycin synergistically enhances chemotherapy-induced cytotoxicity in breast cancer cells. Clin. Cancer Res. 10, 7031–7042 (2004).
Kenny, L. M. et al. Quantification of cellular proliferation in tumor and normal tissues of patients with breast cancer by [18F]fluorothymidine-positron emission tomography imaging: evaluation of analytical methods. Cancer Res. 65, 10104–10112 (2005).
Kenny, L. M. et al. Early assessment of response to treatment in breast cancer by [18F]fluorothymidine-positron emission tomography. J. Clin. Oncol. (Meeting Abstracts) 23, 2084 (2005).
Cleveland, D. W., Mao, Y. & Sullivan, K. F. Centromeres and kinetochores: from epigenetics to mitotic checkpoint signaling. Cell 112, 407–421 (2003).
Acknowledgements
We would like to thank K. Wood, J. Sabry and L. Sauermelch for helpful comments on the manuscript
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors were/are employees of GlaxoSmithKline when writing this Review.
Glossary
- Pharmacodynamic markers
-
A molecular marker of drug response that can be measured in patients receiving the drug. The marker should be a direct measure of modulation of the drug target and be able to show quantitative changes in response to dose.
- Monopolar spindle
-
A mitotic spindle in which the centrosomes are unseparated. A monopolar spindle is incapable of separating the sister chromatids because the spindle poles are not oriented on opposite sides of the chromosomes.
- Spindle tension
-
In a properly functioning mitotic spindle, the microtubules that connect the chromosomes to the centrosomes are under tension. This tension, which creates a pulling force toward the centrosome, can be created by a poleward flux of tubulin within the microtubule, as well as kinesin and dynein motor proteins.
- Bi-orientation
-
This describes chromosomes that have microtubule attatchments to both spindle poles.
- Pharmacodynamic
-
Measurable physiological changes that occur in response to pharmacological modulation.
- Neuropathy
-
A pathological state in which the normal function of the peripheral nervous system is perturbed. In the case of tubulin-binding drugs, this typically manifests as the inhibition of sensory neurons resulting in tingling and/or loss of feeling.
- IC50
-
The concentration of a an inhibitor that is required for 50% inhibition of its target in vitro.
- Immediate early genes
-
Genes whose expression is induced rapidly by growth factor stimulation.
- Mitotic slippage
-
When a cell exits from mitosis without successfully separating the chromosomes or undergoing cytokinesis it is said to have undergone mitotic slippage. Essentially, such a cell has slipped out of mitosis and entered a G1like cell-cycle state but it now has double the chromosome number that it should have in G1.
- Endoreduplication
-
This occurs after a cell undergoes mitotic slippage (having not divided), and then proceeds through the G1 phase with 4N DNA content and enters S phase to replicate its DNA again.
Rights and permissions
About this article
Cite this article
Jackson, J., Patrick, D., Dar, M. et al. Targeted anti-mitotic therapies: can we improve on tubulin agents?. Nat Rev Cancer 7, 107–117 (2007). https://doi.org/10.1038/nrc2049
Issue Date:
DOI: https://doi.org/10.1038/nrc2049
This article is cited by
-
Computational screening identifies depsidones as promising Aurora A kinase inhibitors: extra precision docking and molecular dynamics studies
Network Modeling Analysis in Health Informatics and Bioinformatics (2024)
-
A potent estrogen receptor and microtubule specific purine-benzothiazole-based fluorescent molecular probe induces apoptotic death of breast cancer cells
Scientific Reports (2022)
-
MARK2 regulates chemotherapeutic responses through class IIa HDAC-YAP axis in pancreatic cancer
Oncogene (2022)
-
Phase 1 dose escalation trial of volasertib in combination with decitabine in patients with acute myeloid leukemia
International Journal of Hematology (2021)
-
Chemoradiation impairs myofiber hypertrophic growth in a pediatric tumor model
Scientific Reports (2020)