Key Points
-
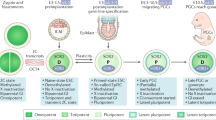
Germ-cell tumours (GCTs) of all anatomical sites can be classified into five groups, characterized by their chromosomal complement and developmental potential.
-
The most significant recurrent chromosomal aberrations in type I yolk-sac tumours are loss of 1p, 4 and 6q, and gain of 1q, 12(p13) and 20q. In type II seminomas and non-seminomatous GCTs, the most significant recurrent chromosomal aberrations are gain of 7, 8, 12p, 21 and X, and loss of chromosomes 1p, 11, 13 and 18. Aberrations of 12p are the only recurrent structural abnormalities in type II GCTs. In type III spermatocytic seminomas, gain of chromosome 9 is most common.
-
The originating cell is most probably a primitive germ cell of which the developmental potential differs according to its stage of maturation and pattern of genomic imprinting.
-
Animal models are available for the different groups of GCTs, except for the type II seminomas and non-seminomatous GCTs.
-
An activating KIT mutation in codon 816 is an early pathogenetic event in bilateral testicular seminomas and non-seminomatous GCTs.
-
The transcription factor OCT3/4, a characteristic of primordial germ cells and pluripotent stem cells, is a new and robust diagnostic marker for type II seminomas and non-seminomatous GCTs, including their intratubular precursor.
-
Treatment sensitivity and resistance of GCTs probably correlates with retention and loss of embryonic characteristics (in particular, DNA-repair deficiency), respectively.
Abstract
The germ-cell tumours are a fascinating group of neoplasms because of their unusual biology and the spectacular therapeutic results that have been obtained in these tumours. Traditionally, this group of neoplasms is presented in an organ-oriented approach. However, recent clinical and experimental data convincingly demonstrate that these neoplasms are one disease with separate entities that can manifest themselves in different anatomical sites. We propose five entities, in which the developmental potential is determined by the maturation stage and imprinting status of the originating germ cell. Recent progress begins to explain the apparent unpredictable development of germ-cell tumours and offers a basis for understanding their exquisite sensitivity to therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Wylie, C. Germ cells. Cell 96, 165–174 (1999).
Zeeman, A. M. et al. VASA is a specific marker for both normal and malignant human germ cells. Lab. Invest. 82, 159–166 (2002).
Oosterhuis, J. W., Looijenga, L. H. J., Van Echten-Arends, J. & De Jong, B. Chromosomal constitution and developmental potential of human germ cell tumors and teratomas. Cancer Genet. Cytogenet. 95, 96–102 (1997). First attempt to classify GCTs according to chromosomal constitution and developmental potential.
Looijenga, L. H. J. & Oosterhuis, J. W. Pathobiology of testicular germ cell tumors: views and news. Analyt. Quant. Cytol. Histol. 24, 263–279 (2002).
Woodward, P. J. et al. in World Health Organization Classification of Tumours. Pathology and Genetics of the Urinary System and Male Genital Organs. (eds. Eble, J. N., Sauter, G., Epstein, J. I. & Sesterhann, I. A.) 217–278 (IARC, Lyon, 2004).
Donovan, P. J. & de Miguel, M. P. Turning germ cells into stem cells. Curr. Opin. Genet. Dev. 13, 463–471 (2003).
Blelloch, R. H. et al. Nuclear cloning of embryonal carcinoma cells. Proc. Natl Acad. Sci. USA 101, 13985–13990 (2004).
Surani, M. A. Reprogramming of genome function through epigenetic inheritance. Nature 414, 122–128 (2001). Critical review of the impact of genomic imprinting on the developmental program of ESCs and EGCs, and its significance for stem-cell transplantation.
McLaren, A. Primordial germ cells in the mouse. Dev. Biol. 262, 1–15 (2003).
McLaren, A. & Southee, D. Entry of mouse embryonic germ cells into meiosis. Dev. Biol. 187, 107–113 (1997). In vitro co-culture experiments showing the dominant role of the microenvironment in making germ cells enter meiosis. The study demonstrates that a testis-specific factor is probably involved in preventing germ cells in the pre-pubertal testis to enter meiosis.
Stallock, J., Molyneaux, K., Schaible, K., Knudson, C. M. & Wylie, C. The pro-apoptotic gene Bax is required for the death of ectopic primordial germ cells during their migration in the mouse embryo. Development 130, 6589–6597 (2003). This study, using fluorescently tagged mouse primordial germ cells in vivo , demonstrated that extragonadal localization leads to a BAX-dependent induction of apoptosis in these cells.
Matsui, Y., Zsebo, K. & Hogan, B. L. Derivation of pluripotential embryonic stem cells from murine primordial germ cells in culture. Cell 70, 841–847 (1992). First publication describing the in vitro conditions that are required to grow mouse primordial germ cells. In vitro , mouse PGCs undergo spontaneous reprogramming to pluripotent embryonal stem cells.
Shamblott, M. J. et al. Derivation of pluripotent stem cells from cultured human primordial germ cells. Proc. Natl Acad. Sci. USA 95, 13726–13731 (1998). First paper on in vitro growth of human primordial germ cells. The cells lose germ-cell characteristics, and undergo spontaneous reprogramming to pluripotent EGCs.
Stevens, L. C. Origin of testicular teratomas from primordial germ cells in mice. J. Natl Cancer Inst. 38, 549–552 (1967). First demonstration that mouse PGCs can give rise to teratomas.
Rescorla, F. J. Pediatric germ cell tumors. Semin. Surg. Oncol. 16, 144–158 (1999).
Gobel, U. et al. Germ-cell tumors in childhood and adolescence. GPOH MAKEI and the MAHO study groups. Ann. Oncol. 11, 263–271 (2000).
Bussey, K. J. et al. SNRPN methylation patterns in germ cell tumors as a reflection of primordial germ cell development. Genes Chromosom. Cancer 32, 342–352 (2001).
Schneider, D. T. et al. Multipoint imprinting analysis indicates a common precursor cell for gonadal and nongonadal pediatric germ cell tumors. Cancer Res. 61, 7268–7276 (2001).
Stevens, L. C. & Little, C. C. Spontaneous testicular teratomas in an inbred strain of mice. Proc. Natl Acad. Sci. USA 40, 1080–1087 (1954).
Stevens, L. C. Experimental production of testicular teratomas in mice of strains 129, A/He, and their F1 hybrids. J. Natl Cancer Inst. 44, 923–929 (1970).
Damjanov, I., Solter, D., Belicza, M. & Skreb, N. Teratomas obtained through extrauterine growth of seven–day old mouse embryos. J. Natl Cancer Inst. 46, 471 (1971).
Solter, D., Dominis, M. & Damjanov, I. Embryo-derived teratocarcinomas: I. The role of strain and gender in the control of teratogenesis. Int. J. Cancer 24, 770–772 (1979).
Walt, H., Oosterhuis, J. W. & Stevens, L. C. Experimental testicular germ cell tumorigenesis in mouse strains with and without spontaneous tumours differs from development of germ cell tumours of the adult human testis. Int. J. Androl. 16, 267–271 (1993).
Stevens, L. C. Development of resistance to teratocarcinogenesis by primordial germ cells in mice. J. Natl Cancer Inst. 37, 859–867 (1966).
Van Berlo, R. J. et al. Yolk-sac carcinoma develops spontaneously as a late occurrence in slow-growing teratoid tumors produced from transplanted 7-day mouse embryos. Int. J. Cancer 45, 153–155 (1990).
Eppig, J. J., Wigglesworth, K., Varnum, D. S. & Nadeau, J. H. Genetic regulation of traits essential for spontaneous ovarian teratocarcinogenesis in strain LT/Sv mice: aberrant meiotic cell cycle, oocyte activation, and parthenogenetic development. Cancer Res. 56, 5047–5054 (1996).
Kimura, T. et al. Conditional loss of PTEN leads to testicular teratoma and enhances embryonic germ cell production. Development 130, 1691–1700 (2003).
Fujishita, T. et al. Development of spontaneous tumours and intestinal lesions in Fhit gene knockout mice. Br. J. Cancer 91, 1571–1574 (2004).
Mostert, M. C. et al. Comparative genomic and in situ hybridization of germ cell tumors of the infantile testis. Lab. Invest. 80, 1055–1064 (2000).
Perlman, E. J. et al. Genetic analysis of childhood endodermal sinus tumors by comparative genomic hybridization. J. Pediatr. Hematol. Oncol. 22, 100–105 (2000).
Veltman, I., Schepens, M. T., Looijenga, L. H. J., Strong, L. C. & Geurts van Kessel, A. Germ cell tumours in neonates and infants: a distinct subgroup. APMIS 111, 152–160 (2003).
Sinke, R. J. et al. Molecular characterization of a recurring complex chromosomal translocation in two human extragonadal germ cell tumors. Cancer Genet. Cytogenet. 73, 11–16 (1994).
Van Echten-Arends, J. et al. Definition of a new entity of malignant extragonadal germ cell tumors. Genes Chromosom. Cancer 12, 8–15 (1995).
Mostofi, F. K., Sesterhenn, I. A. & Davis, C. J. J. Immunopathology of germ cell tumors of the testis. Semin. Diagn. Pathol. 4, 320–341 (1987).
Ulbright, T. M. Germ cell neoplasms of the testis. Am. J. Surg. Pathol. 17, 1075–1091 (1993).
Swerdlow, A. J. in Germ Cell Tumours IV (eds. Jones, W. G., Appleyard, I., Harnden, P. & Joffe, J. K.) 3–8 (John Libbey, London, 1998).
Moul, J. W. et al. Testicular cancer in blacks. A multicenter experience. Cancer 73, 388–393 (1994).
McGregor, J. A. et al. Salivary estriol as risk assessment for preterm labor: a prospective trial. Am. J. Obstet. Gynecol. 173, 1337–1342 (1995).
Henderson, B. E., Bernstein, L., Ross, R. K., Depue, R. H. & Judd, H. L. The early in utero oestrogen and testosteron environment of blacks and whites: potential effects on male offspring. Br. J. Cancer 57, 216–218 (1988).
Skakkebaek, N. E. Testicular dysgenesis syndrome. Horm. Res. 60 (Suppl. 3), 49 (2003).
Skakkebaek, N. E. Testicular dysgenesis syndrome: new epidemiological evidence. Int. J. Androl. 27, 189–191 (2004).
Pugh, R. C. B. in Pathology of the Testis (ed. Pugh, R. C. B.) 245–258 (Blackwell, Oxford, 1976).
Ogunbiyi, J. O., Shittu, O. B., Aghadiuno, P. U. & Lawani, J. Seminoma arising in cryptorchid testes in Nigerian males. East Afr. Med. J. 73, 129–132 (1996).
Skakkebæk, N. E. Possible carcinoma-in-situ of the testis. Lancet II, 516–517 (1972). Paper that identified carcinoma in situ (ITGCNU) as the precursor of testicular GCTs of adolescents and adults.
Loy, V. & Dieckmann, K. P. Carcinoma in situ of the testis: intratubular germ cell neoplasia or testicular intraepithelial neoplasia? Hum. Pathol. 21, 457–458 (1990).
Jacobsen, G. K., Henriksen, O. B. & Van der Maase, H. Carcinoma in situ of testicular tissue adjacent to malignant germ-cell tumors: a study of 105 cases. Cancer 47, 2660–2662 (1981).
Oosterhuis, J. W. et al. Morphology of testicular parenchyma adjacent to germ cell tumours; an interim report. APMIS 111, 32–42 (2003).
Giwercman, A., Müller, J. & Skakkebæk, N. E. Prevalence of carcinoma-in situ and other histopathological abnormalities in testes from 399 men who died suddenly and unexpectedly. J. Urol. 145, 77–80 (1991).
Giwercman, A. & Skakkebæk, N. E. Carcinoma in situ of the testis: biology, screening and management. Eur. Urol. 23, 19–21 (1993).
Møller, H. Decreased testicular cancer risk in men born in wartime. J. Natl Cancer Inst. 81, 1668–1669 (1989). The intra-uterine origin of type II GCTs was supported by the epidemiological observation that men conceived during the Second World War had a reduced risk for testicular GCTs.
Skakkebæk, N. E. et al. Germ cell cancer and disorders of spermatogenesis: an environmental connection? APMIS 106, 3–12 (1998).
Oosterhuis, J. W. et al. Ploidy of primary germ cell tumors of the testis. Pathogenetic and clinical relevance. Lab. Invest. 60, 14–20 (1989).
Oud, P. S. et al. DNA cytometry of pure dysgerminomas of the ovary. Int. J. Gynecol. Pathol. 7, 258–267 (1988).
Rajpert-De Meyts, E. et al. The emerging phenotype of the testicular carcinoma in situ germ cell. APMIS 111, 267–278 (2003).
Van Gurp, R. J. L. M., Oosterhuis, J. W., Kalscheuer, V., Mariman, E. C. M. & Looijenga, L. H. J. Human testicular germ cell tumors show biallelic expression of the H19 and IGF2 gene. J. Natl Cancer Inst. 86, 1070–1075 (1994). The first link between type II GCTs and the germ-cell lineage specific mechanism of genomic imprinting. The tumours showed biallelic expression of imprinted genes, and therefore originated from an erased germ cell.
Albanell, J. et al. Telomerase activity in germ cell cancers and mature teratomas. J. Natl Cancer Inst. 91, 1321–1326 (1999).
Almstrup, K. et al. Embryonic stem cell-like features of testicular carcinoma in situ revealed by genome-wide gene expression profiling. Cancer Res. 64, 4736–4743 (2004).
Palumbo, C. et al. Expression of the PDGF α-receptor 1.5 kb transcript, OCT-4 and c-KIT in human normal and malignant tissues. Implications for early diagnosis of testicular germ cell tumors and understanding regulatory mechanisms. J. Pathol. 196, 467–477 (2002).
Looijenga, L. H. J. et al. POU5F1 (OCT3/4) identifies cells with pluripotent potential in human germ cell tumors. Cancer Res. 63, 2244–2250 (2003).
Gidekel, S., Pizov, G., Bergman, Y. & Pikarsky, E. Oct-3/4 is a dose-dependent oncogenic fate determinant. Cancer Cell 4, 361–370 (2003).
Sperger, J. M. et al. Gene expression patterns in human embryonic stem cells and human pluripotent germ cell tumors. Proc. Natl Acad. Sci. USA 100, 13350–13355 (2003). The first large expression-profiling study on human type II GCTs showing similarities between primordial germ cells and seminoma. Genes on chromosome 12p showed overexpression in embryonal carcinoma compared with embryonic stem cells.
Jones, T. D., Ulbright, T. M., Eble, J. N., Baldridge, L. A. & Cheng, L. OCT4 staining in testicular tumors: a sensitive and specific marker for seminoma and embryonal carcinoma. Am. J. Surg. Pathol. 28, 935–940 (2004).
Rajpert-De Meyts, E. et al. Developmental expression of POU5F1 (OCT-3/4) in normal and dysgenetic human gonads. Hum. Reprod. 19, 1338–1344 (2004).
Cheng, L. et al. OCT4: A novel biomarker for dysgerminoma of the ovary. Am. J. Surg. Pathol. 28, 1341–1346 (2004).
Niwa, H., Miyazaki, J. & Smith, A. G. Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nature Genet. 24, 372–376 (2000). The crucial role of OCT3/4 in determining possible differentiation lineages is demonstrated in mouse embryonic stem cells. The level of expression is very strictly correlated with the differentiation status and lineage of the cell.
Hay, D. C., Sutherland, L., Clark, J. & Burdon, T. Oct-4 knockdown induces similar patterns of endoderm and trophoblast differentiation markers in human and mouse embryonic stem cells. Stem Cells 22, 225–235 (2004).
Liu, L., Czerwiec, E. & Keefe, D. L. Effect of ploidy and parental genome composition on expression of Oct-4 protein in mouse embryos. Gene Expr. Patterns 4, 433–441 (2004).
Kehler, J. et al. Oct4 is required for primordial germ cell survival. EMBO Rep. 5, 1078–1083 (2004).
Honecker, F. et al. Pathobiological implications of the expression of markers of testicular carcinoma in situ by foetal germ cells. J. Pathol. 203, 849–857 (2004).
Stoop, H. et al. Differentiation and development of human female germ cells during prenatal gonadogenesis: an immunohistochemical stdy. Hum. Reprod. (in the press).
Matin, M. M. et al. Specific knockdown of Oct4 and β2-microglobulin expression by RNA interference in human embryonic stem cells and embryonic carcinoma cells. Stem Cells 22, 659–668 (2004).
Kersemaekers, A. M. et al. Identifies of germ cells at risk for neoplastic transformation in gonadoblastomas: an immunohistochemical study for OCT3/4 and TSPY. Hum. Pathol. (in the press).
Scully, R. E. Gonadoblastoma: a review of 74 cases. Cancer 25, 1340–1356 (1970).
Lau, Y., Chou, P., Iezzoni, J., Alonzo, J. & Komuves, L. Expression of a candidate gene for the gonadoblastoma locus in gonadoblastoma and testicular seminoma. Cytogenet. Cell Genet. 91, 160–164 (2000).
Deb-Rinker, P., Ly, D., Jezierski, A., Sikorska, M. & Walker, P. R. Sequential DNA methylation of the Nanog and Oct-4 upstream regions in human NT2 cells during neuronal differentiation. J. Biol. Chem. 21 Dec 2004 (10.1074/jbc.C400479200).
Hattori, N. et al. Epigenetic control of mouse Oct-4 gene expression in embryonic stem cells and trophoblast stem cells. J. Biol. Chem. 279, 17063–17069 (2004).
Skakkebaek, N. E. et al. Germ cell cancer and disorders of spermatogenesis: an environmental connection? APMIS 106, 3–11 (1998).
Donner, J., Kliesch, S., Brehm, R. & Bergmann, M. From carcinoma in situ to testicular germ cell tumour. APMIS 112, 79–88 (2004).
Von Eyben, F. E., Jacobsen, G. K., Rorth, M. & Von Der Maase, H. Microinvasive germ cell tumour (MGCT) adjacent to testicular germ cell tumours. Histopathology 44, 547–554 (2004).
Walsh, J. & Andrews, P. W. Expression of Wnt and Notch pathway genes in a pluripotent human embryonal carcinoma cell line and embryonic stem cell. APMIS 111, 197–210 (2003).
Looijenga, L. H. J., Gillis, A. J. M., Van Gurp, R. J. H. L. M., Verkerk, A. J. M. H. & Oosterhuis, J. W. X inactivation in human testicular tumors. XIST expression and androgen receptor methylation status. Am. J. Pathol. 151, 581–590 (1997).
Kawakami, T., Okamoto, K., Ogawa, O. & Okada, Y. XIST unmethylated DNA fragments in male-derived plasma as a tumour marker for testicular cancer. Lancet 363, 40–42 (2004).
Geurts van Kessel, A. et al. Chromosome 12q heterozygosity is retained in i(12p)-positive testicular germ cell tumor cells. Cancer Genet. Cytogenet. 40, 129–134 (1989).
Mayer, F. et al. Aneuploidy of human testicular germ cell tumors is associated with amplification of centrosomes. Oncogene 22, 3859–3866 (2003).
Galitski, T., Saldanha, A. J., Styles, C. A., Lander, E. S. & Fink, G. R. Ploidy regulation of gene expression. Science 285, 251–254 (1999).
Comai, L. et al. Phenotypic instability and rapid gene silencing in newly formed arabidopsis allotetraploids. Plant Cell 12, 1551–1568 (2000).
Oosterhuis, J. W. et al. Ploidy of malignant mediastinal germ-cell tumors. Hum. Pathol. 21, 729–732 (1990).
Riopel, M. A., Spellerberg, A., Griffin, C. A. & Perlman, E. J. Genetic analysis of ovarian germ cell tumors by comparative genomic hybridization. Cancer Res. 58, 3105–3110 (1998).
Kraggerud, S. M. et al. DNA copy number changes in malignant ovarian germ cell tumors. Cancer Res. 60, 3025–3030 (2000).
Van Echten-Arends, J. et al. No recurrent structural abnormalities in germ cell tumors of the adult testis apart from i(12p). Genes Chromosom. Cancer 14, 133–144 (1995).
Hoglund, M. et al. Multivariate analyses of genomic imbalances in solid tumors reveal distinct and converging pathways of karyotypic evolution. Genes Chromosom. Cancer 31, 156–171 (2001).
Frigyesi, A. et al. A model for karyotypic evolution in testicular germ cell tumors. Genes Chromosom. Cancer 40, 172–178 (2004).
Castedo, S. M. M. J. et al. Cytogenetic analysis of ten human seminomas (two of them lacking the i(12p)). Cancer Res. 49, 439–443 (1989).
Rodriguez, E. et al. Cytogenetic analysis of 124 prospectively ascertained male germ cell tumors. Cancer Res. 52, 2285–2291 (1993).
Ottesen, A. M. et al. Detection of chromosomal aberrations in seminomatous germ cell tumours using comparative genomic hybridization. Genes Chromosom. Cancer 20, 412–418 (1997).
Summersgill, B. et al. Molecular cytogenetic analysis of adult testicular germ cell tumours and identification of regions of consensus copy number change. Br. J. Cancer 77, 305–313 (1998).
Looijenga, L. H. J. et al. Comparative genomic hybridization of microdissected samples from different stages in the development of a seminoma and nonseminoma. J. Pathol. 19, 187–192 (2000).
Kraggerud, S. M. et al. Genome profiles of familial/bilateral and sporadic testicular germ cell tumors. Genes Chromosom. Cancer 34, 168–174 (2002).
Chaganti, R. S. K., Rodriguez, E. & Mathew, S. Origin of adult male mediastinal germ-cell tumours. Lancet 343, 1130–1132 (1994).
Rapley, E. A., Crockford, G. P., Easton, D. F., Stratton, M. R. & Bishop, D. T. Localisation of susceptibility genes for familial testicular germ cell tumour. APMIS 111, 128–133 (2003).
Rapley, E. A. et al. Localization to Xq27 of a susceptibility gene for testicular germ-cell tumours. Nature Genet. 24, 197–200 (2000).
von Eyben, F. E. Chromosomes, genes, and development of testicular germ cell tumors. Cancer Genet. Cytogenet. 151, 93–138 (2004).
Atkin, N. B. & Baker, M. C. Specific chromosome change, i(12p), in testicular tumours? Lancet 2, 1349 (1982). First demonstration, by conventional kayotyping, of i(12p) as specific chromosomal aberration in testicular GCTs. Subsequently, i(12p) was demonstrated by others in GCTs of similar histology in the ovary, the mediastinum and the midline of the brain (type II GCTs).
Looijenga, L. H. J. et al. Role of gain of 12p in germ cell tumour development. APMIS 111, 161–173 (2003).
Suijkerbuijk, R. F. et al. Overrepresentation of chromosome 12p sequences and karyotypic evolution in i(12p)-negative testicular germ-cell tumors revealed by fluorescence in situ hybridization. Cancer Genet. Cytogenet. 70, 85–93 (1993).
Rodriguez, E. et al. Molecular cytogenetic analysis of i(12p)-negative human male germ cell tumors. Genes Chromosom. Cancer 8, 230–236 (1993).
Koul, S. et al. Characteristic promoter hypermethylation signatures in male germ cell tumors. Mol. Cancer 1, 8 (2002).
Smith-Sorensen, B. et al. Frequent promoter hypermethylation of the O6-methylguanine-DNA methyltransferase (MGMT) gene in testicular cancer. Oncogene 21, 8878–8884 (2002).
Smiraglia, D. J. et al. Distinct epigenetic phenotypes in seminomatous and nonseminomatous testicular germ cell tumors. Oncogene 21, 3909–3916 (2002).
Honorio, S. et al. Frequent epigenetic inactivation of the RASSF1A tumour suppressor gene in testicular tumours and distinct methylation profiles of seminoma and nonseminoma testicular germ cell tumours. Oncogene 22, 461–466 (2003).
Skotheim, R. I. et al. New insights into testicular germ cell tumorigenesis from gene expression profiling. Cancer Res. 62, 2359–2364 (2002).
Houldsworth, J., Reuter, V., Bosl, G. J. & Chaganti, R. S. K. Aberrant expression of cyclin D2 is an early event in human male germ cell tumorigenesis. Cell Growth Differ. 8, 293–299 (1997).
Draper, J. S. et al. Recurrent gain of chromosomes 17q and 12 in cultured human embryonic stem cells. Nature Biotechnol. 22, 53–54 (2004).
Skotheim, R. I. et al. Candidate genes for testicular cancer evaluated by in situ protein expression analyses on tissue microarrays. Neoplasia 5, 397–404 (2003).
De Graaff, W. E. et al. Ploidy of testicular carcinoma in situ. Lab. Invest. 66, 166–168 (1992).
Rosenberg, C., Van Gurp, R. J., Geelen, E., Oosterhuis, J. W. & Looijenga, L. H. Overrepresentation of the short arm of chromosome 12 is related to invasive growth of human testicular seminomas and nonseminomas. Oncogene 19, 5858–5862 (2000). This study shows that invasive type II GCTs consistently show over-representation of the short arm of chromosome 12 and that their common precursor lesion, ITGCNU, lacks it. Over-representation of 12p is apparently related to progression of the cancer.
Summersgill, B., Osin, P., Lu, Y. J., Huddart, R. & Shipley, J. Chromosomal imbalances associated with carcinoma in situ and associated testicular germ cell tumours of adolescents and adults. Br. J. Cancer 85, 213–220 (2001).
Ottesen, A. M. et al. High-resolution comparative genomic hybridization detects extra chromosome arm 12p material in most cases of carcinoma in situ adjacent to overt germ cell tumors, but not before the invasive tumor development. Genes Chromosom. Cancer 38, 117–125 (2003).
Oosterhuis, J. W. & Looijenga, L. H. J. The biology of human germ cell tumours: retrospective speculations and new prospectives. Eur. Urol. 23, 245–250 (1993).
Rao, P. H. et al. Chromosomal amplification is associated with cisplatinum resistance of human male germ cell tumors. Cancer Res. 58, 4260–4263 (1998).
Roelofs, H. et al. Restricted 12p-amplification and RAS mutation in human germ cell tumors of the adult testis. Am. J. Pathol. 157, 1155–1166 (2000).
Zafarana, G. et al. Coamplification of DAD-R, SOX5, and EKI1 in human testicular seminomas, with specific overexpression of DAD-R, correlates with reduced levels of apoptosis and earlier clinical manifestation. Cancer Res. 62, 1822–1831 (2002).
Olie, R. A. et al. N- and KRAS mutations in human testicular germ cell tumors: incidence and possible biological implications. Genes Chromosom. Cancer 12, 110–116 (1995).
Bartkova, J., Rajpert-De Meyts, E., Skakkebaek, N. E. & Bartek, J. D-type cyclins in adult human testis and testicular cancer: relation to cel type, proliferaton, differentiation, and malignancy. J. Pathol. 187, 573–581 (1999).
Rodriguez, S. et al. Expression profile of genes from 12p in testicular germ cell tumors of adolescents and adults associated with i(12p) and amplification at 12p11. 2-p12. 1. Oncogene 22, 1880–1891 (2003).
Bourdon, V. et al. Genomic and expression analysis of the 12p11-p12 amplicon using EST arrays identifies two novel amplified and overexpressed genes. Cancer Res. 62, 6218–6223 (2002).
Zafarana, G. et al. 12p-amplicon structure analysis in testicular germ cell tumors of adolescents and adults by array-CGH. Oncogene 22, 7695–7701 (2003).
Clark, A. T. et al. Human STELLAR, NANOG, and GDF3 genes are expressed in pluripotent cells and map to chromosome 12p13, a hotspot for teratocarcinoma. Stem Cells 22, 169–179 (2004).
Mostert, M. C. et al. Identification of the crucial region of 12p overrepresentation in testicular germ cell tumors of adolescents and adults. Oncogene 16, 2617–2627 (1998).
Henegariu, O. et al. Characterization of gains, losses, and regional amplification in testicular germ cell tumor cell lines by comparative genomic hybridization. Cancer Genet. Cytogenet. 148, 14–20 (2004).
McIntyre, A. et al. Defining minimum genomic regions of imbalance involved in testicular germ cell tumors of adolescents and adults through genome wide microarray analysis of cDNA clones. Oncogene 23, 9142–9147 (2004).
Heidenblad, M. et al. Detailed genomic mapping and expression analyses of 12p amplifications in pancreatic carcinomas reveal a 3. 5-Mb target region for amplification. Genes Chromosom. Cancer 34, 211–223 (2002).
De Miguel, M. P., Cheng, L., Holland, E. C., Federspiel, M. J. & Donovan, P. J. Dissection of the c-Kit signaling pathway in mouse primordial germ cells by retroviral-mediated gene transfer. Proc. Natl Acad. Sci. USA 99, 10458–10463 (2002). Using a novel technical approach, the signalling pathways in mouse germ cells could be investigated in detail. The AKT kinase was found to be important for growth in vitro.
Rajpert-De Meyts, E. & Skakkebæk, N. E. Expression of the c-kit protein product in carcinoma-in-situ and invasive testicular germ cell tumours. Int. J. Androl. 17, 85–92 (1994).
Donovan, P. J. Growth factor regulation of mouse primordial germ cell development. Curr. Top. Dev. Biol. 29, 189–225 (1994).
Olie, R. A. et al. in Advances in Biosciences Vol 91 Germ Cell Tumors III (eds. Jones, W. G., Harnden, P. & Appleyard, I.) 95–106 (Pergamon, 1994).
Tian, Q., Frierson, H. F. Jr., Krystal, G. W. & Moskaluk, C. A. Activating c-kit gene mutations in human germ cell tumors. Am. J. Pathol. 154, 1643–1647 (1999).
Kemmer, K. et al. KIT mutations are common in testicular seminomas. Am J. Pathol. 164, 305–313 (2004).
Looijenga, L. H. J. et al. Stem cell factor receptor (c-KIT) codon 816 mutations predict development of bilateral testicular germ cell tumors. Cancer Res. 63, 7674–7678 (2003).
Dieckmann, K. P. & Loy, V. Prevalence of contralateral testicular intraepithelial neoplasia in patients with testicular germ cell neoplasms. J. Clin. Oncol. 14, 3126–3132 (1996).
Lajos, G., Gomez, F., Mihaly, B. & Istvan, B. The incidence, prognosis, clinical and histological characteristics, treatment and outcome of patients with bilateral germ cell testicular cancer in Hungary. J. Cancer Res. Clin. Oncol. 129, 309–315 (2003).
Rorth, M. et al. Carcinoma in situ of the testis. Scan. J. Urol. 205, 166–186 (2000).
Gaffan, J. et al. Infertility rates following POMB/ACE chemotherapy for male and female germ cell tumours — a retrospective long-term follow-up study. Br. J. Cancer 89, 1849–1854 (2003).
De Gouveia Brazao, C. A. et al. Bilateral testicular microlithiasis predicts development of malignant testicular germ cell tumours in subfertile men. J. Urol. 171, 158–160 (2004).
Holm, M., Hoei-Hansen, C. E., Rajpert-De Meyts, E. & Skakkebaek, N. E. Increased risk of carcinoma in situ in patients with testicular germ cell cancer with ultrasonic microlithiasis in the contralateral testicle. J. Urol. 170, 1163–1167 (2003).
Heidenreich, A. & Moul, J. W. Contralateral testicular biopsy procedure in patients with unilateral testis cancer: is it indicated? Semin. Urol. Oncol. 20, 234–238 (2002).
Oosterhuis, J. W., Andrews, P. W., Knowles, B. B. & Damjanov, I. Effects of cis-platinum on embryonal carcinoma cell lines in vitro. Int. J. Cancer 34, 133–139 (1984).
Cairns, J. Somatic stem cells and the kinetics of mutagenesis and carcinogenesis. Proc. Natl Acad. Sci. USA 99, 10567–10570 (2002).
Van Sloun, P. P. et al. The role of nucleotide excision repair in protecting embryonic stem cells from genotoxic effects of UV-induced DNA damage. Nucleic Acids Res. 27, 3276–3282 (1999).
Hong, Y. & Stambrook, P. J. Restoration of an absent G1 arrest and protection from apoptosis in embryonic stem cells after ionizing radiation. Proc. Natl Acad. Sci. USA 101, 14443–14448 (2004).
Kersemaekers, A. M. F. et al. Role of p53 and MDM2 in treatment response of human germ cell tumors. J. Clin. Oncol. 20, 1551–1561 (2002).
Mayer, F. et al. p53 and MDM2 in germ cell cancer treatment response. J. Clin. Oncol. 20, 3928–3929 (2002).
Masters, J. R. & Koberle, B. Curing metastatic cancer: lessons from testicular germ-cell tumours. Nature Rev. Cancer 3, 517–525 (2003). A comprehensive review on treatment sensitivity and resistance of type II GCTs. A link to embryonal characteristics of these tumours is made, and the role of p53 is questioned.
Rajpert-De Meyts, E. et al. The immunohistochemical expression pattern of Chk2, p53, p19INK4d, MAGE-A4 and other selected antigens provides new evidence for the premeiotic origin of spermatocytic seminoma. Histopathology 42, 217–226 (2003).
Koberle, B., Masters, J. R., Hartley, J. A. & Wood, R. D. Defective repair of cisplatin-induced DNA damage caused by reduced XPA protein in testicular germ cell tumours. Curr. Biol. 9, 273–276 (1999).
Honecker, F. et al. Xeroderma pigmentosum group A protein and chemotherapy-resistamnce in human germ cell tumors. Lab. Invest. 83, 1489–1495 (2003).
Zamble, D. B., Mikata, Y., Eng, C. H., Sandman, K. E. & Lippard, S. J. Testis-specific HMG-domain protein alters the responses of cells to cisplatin. J. Inorg. Biochem. 91, 451–462 (2002).
Spierings, D. C., de Vries, E. G., Vellenga, E. & de Jong, S. The attractive Achilles heel of germ cell tumours: an inherent sensitivity to apoptosis-inducing stimuli. J. Pathol. 200, 137–148 (2003).
Schenk, P. W. et al. Resistance to platinum-containing chemotherapy in testicular germ cell tumours is associated with downregulation of the protein kinase SRPK1. Neoplasia 6, 297–301 (2004).
International Germ Cell Consensus Classification: a prognostic factor- based staging system for metastatic germ cell cancers. International Germ Cell Cancer Collaborative Group. J. Clin. Oncol. 15, 594–603 (1997).
Bokemeyer, C. et al. Early prediction of treatment response to high-dose salvage chemotherapy in patients with relapsed germ cell cancer using [18F]FDG PET. Br. J. Cancer 86, 506–511 (2002).
Bartkova, J., Thullberg, M., Rajpert-De Meyts, E., Skakkebaek, N. E. & Bartek, J. Cell cycle regulators in testicular cancer: loss of p18INK4C marks progression from carcinoma in situ to invasive germ cell tumours. Int. J. Cancer 85, 370–375 (2000).
Fan, S. et al. Cells lacking CIP1/WAF1 genes exhibit preferential sensitivity to cisplatin and nitrogen mustard. Oncogene 14, 2127–2136 (1997).
Oosterhuis, J. W. et al. Effects of multiple-drug chemotherapy (cis-diammine- dichloroplatinum, bleomycin, and vinblastine) on the maturation of retroperitoneal lymph node metastases of nonseminomatous germ cell tumors of the testis. Cancer 51, 408–416 (1983).
Mayer, F. et al. Microsatellite instability of germ cell tumors is associated with resistance to systemic treatement. Cancer Res. 62, 2758–2760 (2002).
Mueller, T. et al. Failure of activation of caspase-9 induces a higher threshold for apoptosis and cisplatin resistance in testicular cancer. Cancer Res. 63, 513–521 (2003).
Mayer, F. et al. Molecular determinants of treatment response in human germ cell tumors. Clin. Cancer Res. 9, 767–773 (2003).
Timmer-Bosscha, H., de Vries, E. G., Meijer, C., Oosterhuis, J. W. & Mulder, N. H. Differential effects of all-trans-retinoic acid, docosahexaenoic acid, and hexadecylphosphocholine on cisplatin-induced cytotoxicity and apoptosis in a cisplantin-sensitive and resistant human embryonal carcinoma cell line. Cancer Chemother. Pharmacol. 41, 469–476 (1998).
Bartkova, J., Thullberg, M., Rajpert-De Meyts, E., Skakkebaek, N. E. & Bartek, J. Lack of p19INK4d in human testicular germ-cell tumours contrasts with high expression during normal spermatogenesis. Oncogene 19, 4146–4150 (2000).
Bartkova, J., Rajpert-De Meyts, E., Skakkebæk, N. E., Lukas, J. & Bartek, J. Deregulation of the G1/S-phase control in human testicular germ cell tumours. APMIS 111, 252–265 (2003).
Cummings, O. W., Ulbright, T. M., Eble, J. N. & Roth, L. M. Spermatocytic seminoma: an immunohistochemical study. Hum. Pathol. 25, 54–59 (1994).
Eble, J. N. Spermatocytic seminoma. Hum. Pathol. 25, 1035–1042 (1994).
Chung, P. W. et al. Spermatocytic seminoma: a review. Eur. Urol. 45, 495–498 (2004).
Dekker, I., Rozeboom, T., Delemarre, J., Dam, A. & Oosterhuis, J. W. Placental-like alkaline phosphatase and DNA flow cytometry in spermatocytic seminoma. Cancer 69, 993–996 (1992).
Stoop, H. et al. Reactivity of germ cell maturation stage-specific markers in spermatocytic seminoma: diagnostic and etiological implications. Lab. Invest. 81, 919–928 (2001).
Rajpert-De Meyts, E. et al. The immunohistochemical expression pattern of Chk2, p53, p19INK4d, MAGE-A4 and other selected antigens provides new evidence for the premeiotic origin of spermatocytic seminoma. Histopathology 42, 217–226 (2003).
Rosenberg, C. et al. Chromosomal constitution of human spermatocytic seminomas: comparative genomic hybridization supported by conventional and interphase cytogenetics. Genes Chromosom. Cancer 23, 286–291 (1998).
Looijenga, L. H. J. et al. Seminomas of the canine testis; counterpart of spermatocytic seminoma of men? Lab. Invest. 71, 490–496 (1994).
Subramaniam, K. & Seydoux, G. Dedifferentiation of primary spermatocytes into germ cell tumors in C. elegans lacking the pumilio-like protein PUF-8. Curr. Biol. 13, 134–139 (2003).
Rosenberg, C. et al. Chromosomal constitution of human spermatocytic seminomas: comparative genomic hybridization suppored by conventional and interphase cytogenetics. Genes Chromosom. Cancer 23, 286–291 (1998).
Verkerk, A. J. et al. Unique expression patterns of H19 in human testicular cancers of different etiology. Oncogene 14, 95–107 (1997).
Durcova-Hills, G., Burgoyne, P. & McLaren, A. Analysis of sex differences in EGC imprinting. Dev. Biol. 268, 105–110 (2004).
Hajkova, P. et al. Epigenetic reprogramming in mouse primordial germ cells. Mech. Dev. 117, 15–23 (2002).
Wilkins, J. F. & Haig, D. What good is genomic imprinting: the function of parent-specific gene expression. Nature Rev. Genet. 4, 359–368 (2003).
Walter, J. & Paulsen, M. Imprinting and disease. Semin. Cell Dev. Biol. 14, 101–110 (2003).
Allegrucci, C., Denning, C., Priddle, H. & Young, L. Stem-cell consequences of embryo epigenetic defects. Lancet 364, 206–208 (2004).
McGrath, J. & Solter, D. Nuclear transplantation in mouse embryos. J. Exp. Zool. 228, 355–362 (1983). The functional difference between some of the parental chromosomes, now known as genomic imprinting, was demonstrated by exchanging haploid sets of maternal and paternal chromosomes in mouse zygotyes. It was shown that a bimaternal imprinting (gynogenote) pattern favours somatic differentiation, and a bipaternal pattern favours trophoblastic differentiation.
Surani, M. A., Barton, S. C. & Norris, M. L. Nuclear transplantation in the mouse: heritable differences between parental genomes after activation of the embryonic genome. Cell 45, 127–136 (1986).
Oosterhuis, J. W. & Looijenga, L. H. Current views on the pathogenesis of testicular germ cell tumours and perspectives for future research: highlights of the 5th Copenhagen Workshop on Carcinoma In Situ and Cancer of the Testis. APMIS 111, 280–289 (2003).
Bredael, J. J., Vugrin, D. & Whitmore, W. F. J. Autopsy findings in 154 patients with germ cell tumors of the testis. Cancer 50, 548–551 (1982).
Acknowledgements
The authors would like to thank the many collaborators involved in our own studies refered to in this review, as well as the urologists and pathologists who actively participate by providing tissues. The Dutch Cancer Society is acknowledged for its financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- RETROPERITONEAL
-
Located at the back of the abdomen, behind the lining known as the peritoneum that covers organs such as the stomach, liver, and parts of the large and small intestine. Organs such as the pancreas and kidneys are retroperitoneal.
- MEDIASTINAL
-
Anatomical space of the thorax. Organs such as the thymus and heart are found within the mediastinum.
- YOLK-SAC TUMOUR
-
Subtype of the non-seminomatous germ-cell tumour that histologically resembles the yolk sac. It can appear as a pure tumour or as a part of a mixed non-seminomatous germ-cell tumour.
- EPIBLAST
-
Columnar epithelium that lines the floor of the amniotic sac. This layer generates endoderm and mesoderm by migration of cells through the primitive streak. The remaining cells form ectoderm.
- GENITAL RIDGES
-
A paired fold in the dorsal lining of the abdominal cavity of the embryo in which the gonads develop.
- HYDATIDIFORM MOLE
-
Benign gestational neoplasm, grossly presenting as a bunch of grapes.
- PARTHENOGENETIC OOCYTES
-
Oocytes that progress beyond meiosis I without being fertilized.
- CRYPTORCHIDISM
-
The failure of the testes to descend into the scrotum.
- GONADAL DYSGENESIS
-
Defective development of the gonads such as that seen in Turner's syndrome, where patients have only one complete X chromosome.
- EMBRYOID BODIES
-
Cellular structures that arise when embryonic stem cells are cultured in vitro; they contain tissues from all three germ layers — endoderm, mesoderm and ectoderm.
- ISOCHROMOSOME
-
An abnormal chromosome having a median centromere and two identical arms.
- DESMOSOME
-
An adhesive junction that anchors intermediate filaments between adjoining cells.
- INTERMEDIATE JUNCTIONS
-
Intermediate junctions are made of thickened cell membranes; the extra thickness is caused by polysaccharide, mostly hyaluronic acid, gluing the membranes together.
- MICROLITHIASIS
-
The formation of minute deposits of mainly inorganic material in tissues; for example, in the seminiferous tubules of the testis.
- GYNOGENOTES
-
Animals with maternal chromosomes only that are artificially created using nuclear transfer experiments. Experimental gynogenotes support outgrowth of the somatic tissues of the embryo proper.
- ANDROGENOTES
-
Animals with paternal chromosomes only that are artificially created using nuclear transfer experiments. Androgenotes support the development of the trophoblast.
Rights and permissions
About this article
Cite this article
Oosterhuis, J., Looijenga, L. Testicular germ-cell tumours in a broader perspective. Nat Rev Cancer 5, 210–222 (2005). https://doi.org/10.1038/nrc1568
Issue Date:
DOI: https://doi.org/10.1038/nrc1568
This article is cited by
-
A rapid and stable spontaneous reprogramming system of Spermatogonial stem cells to Pluripotent State
Cell & Bioscience (2023)
-
Characterizing the mutational burden, DNA methylation landscape, and proteome of germ cell tumor-related somatic-type malignancies to identify the tissue-of-origin, mechanisms of therapy resistance, and druggable targets
British Journal of Cancer (2023)
-
Subsets of preoperative sex hormones in testicular germ cell cancer: a retrospective multicenter study
Scientific Reports (2023)
-
Genome-scale CRISPR screen reveals neddylation to contribute to cisplatin resistance of testicular germ cell tumours
British Journal of Cancer (2023)
-
Somatische Malignitäten des Hodens
Die Pathologie (2023)