Key Points
-
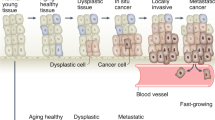
The promise of early detection is that it will identify cancer while still localized and curable, preventing not only mortality, but also reducing morbidity and costs.
-
Cervical cancer is a historical illustration of the promise of early detection; countries with broad screening programmes have markedly reduced disease-related deaths. However, the efficacy and practicality of screening tests for most other cancers remain controversial.
-
The advent of new technologies — including transcript (gene-expression) analysis, genomic DNA-based methods and proteomics — offer many new opportunities for developing biomarker-based tests that are less expensive and more accurate than existing screening tests.
-
To develop and fully evaluate a new screening test requires attention to all phases of biomarker development, including identification of promising biomarkers, production of assays that can detect both clinical and pre-clinical disease, development of tests that combine sensitive biomarkers to achieve greater diagnostic accuracy, and evaluation of the impact of the tests on disease mortality and costs.
-
With many potential biomarkers in the early-detection pipeline, it will be important to develop strategies for evaluating the benefits and costs of biomarker-based tests within a reasonable time frame.
-
The dissemination of screening tests that have been inadequately evaluated can have grave consequences, including invasive follow-up of healthy individuals, morbidity from unnecessary treatment and vastly increased costs to the medical system. Although randomized screening trials remain the ultimate test of screening efficacy in preventing disease-specific mortality, it will be important to develop these and other analytical approaches so that inferences about screening costs and benefits can be made in an efficient and timely fashion.
Abstract
Early detection represents one of the most promising approaches to reducing the growing cancer burden. It already has a key role in the management of cervical and breast cancer, and is likely to become more important in the control of colorectal, prostate and lung cancer. Early-detection research has recently been revitalized by the advent of novel molecular technologies that can identify cellular changes at the level of the genome or proteome, but how can we harness these new technologies to develop effective and practical screening tests?
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
National Cancer Institute. Surveillance Epidemiology and End Results Program [online], (cited 19 Nov 2002), <http://seer.cancer.gov/> (2002).
Winawer, S. J. et al. Prevention of colorectal cancer: guidelines based on new data. WHO Collaborating Center for the Prevention of Colorectal Cancer. Bull. World Health Organ. 73, 7–10 (1995).
Bell, R., Petticrew, M. & Sheldon, T. The performance of screening tests for ovarian cancer: results of a systematic review. Br. J. Obst. Gynaecol. 105, 1136–1147 (1998).
Henschke, C. Early Lung Cancer Action Project: overall design and findings from baseline screening. Cancer 89, 274–282 (2000).
Henschke, C. et al. Early Lung Cancer Action Project: initial findings on repeat screening. Cancer 92, 153–159 (2001).
Mahadevia, P. J. et al. Lung cancer screening with helical computed tomography in older adult smokers: a decision and cost-effectiveness analysis. JAMA 289, 313–322 (2003).
Grann, V. R. & Neugut, A. I. Lung cancer screening at any price? JAMA 289, 357–358 (2003).
Etzioni, R. et al. Overdiagnosis due to prostate-specific antigen screening: lessons from US prostate cancer incidence trends. J. Natl Cancer Inst. 94, 981–990 (2002).
Petricoin, E. F. et al. Use of proteomic patterns in serum to identify ovarian cancer. Lancet 359, 572–577 (2002). This article illustrates the potential utility of serum proteomics in developing sensitive and specific cancer screening tests.
Dhanasekaran, S. M. et al. Delineation of prognostic biomarkers in prostate cancer. Nature 412, 822–826 (2001).
Pepe, M. S. et al. Phases of biomarker development for early detection of cancer. J. Natl Cancer Inst. 93, 1054–1061 (2001). First paper to propose the classification of biomarker studies into five phases.
Pepe, M. S., Longton, G. M., Anderson, G. L. & Schummer, M. Selecting Differentially Expressed Genes from Microarray Experiments UW Biostatistics Working Paper Series. Working Paper 184 [online], (cited 24 Jan 2003), <http://www.bepress.com/uwbiostat/paper184> (2003).
Tusher, V. G., Tibshirani, R. & Chu, G. Significance analysis of microarrays applied to the ionizing radiation response. Proc. Natl Acad. Sci. USA 98, 5116–5121 (2001).
Yeung, K. Y., Haynor, D. R. & Ruzzo, W. L. Validating clustering for gene expression data. Bioinformatics 17, 309–318 (2001).
Yeung, K. Y., Fraley, C., Murua, A., Raftery, A. E. & Ruzzo, W. L. Model-based clustering and data transformations for gene expression data. Bioinformatics 17, 977–987 (2001).
Hastie, T., Tibshirani, R. & Friedman, J. The Elements of Statistical Learning: Data Mining, Inference and Prediction (Springer–Verlag, New York, 2001).
Gann, P. H., Hennekens, C. H. & Stampfer, M. J. A prospective evaluation of plasma prostate-specific antigen for detection of prostatic cancer. JAMA 273, 289–294 (1995).
McIntosh, M., Urban, N. & Karlan, B. Generating longitudinal screening algorithms using novel biomarkers for disease. Cancer Epidemiol. Biomarkers Prev. 11, 159–166 (2002).
Carter, H. B. & Pearson, J. D. Prostate-specific antigen velocity and repeated measures of prostate-specific antigen. Urol. Clin. N. Am. 24, 333–338 (1997).
Polascik, T. J., Oesterling, J. E. & Partin, A. W. Prostate specific antigen: a decade of discovery — what we have learned and where we are going. J. Urol. 162, 293–306 (1999).
Skates, C. J., Pauler, D. K. & Jacobs, I. Screening based on the risk of cancer calculation from Bayesian hierarchical change-point models of longitudinal markers. J. Am. Stat. Assoc. 96, 429–439 (2001).
Carter, H. B. et al. Estimation of prostatic growth using serial prostate-specific antigen measurements in men with and without prostate disease. Cancer Res. 52, 3323–3328 (1992).
Woods, W. G. et al. Screening of infants and mortality due to neuroblastoma. N. Engl. J. Med. 346, 1041–1046 (2002).
Farrow, D. C. & Vaughan, T. L. Determinants of survival following the diagnosis of esophageal adenocarcinoma (United States). Cancer Causes Control 7, 322–327 (1996).
Conio, M. et al. Secular trends in the epidemiology and outcome of Barrett's oesophagus in Olmsted County, Minnesota. Gut 48, 304–309 (2001).
Drewitz, D. J., Sampliner, R. E. & Garewal, H. S. The incidence of adenocarcinoma in Barrett's esophagus: a prospective study of 170 patients followed for 8 years. Am. J. Gastroenterol. 92, 212–215 (1997).
Schnell, T. G. et al. Long-term nonsurgical management of Barrett's esophagus with high-grade dysplasia. Gastroenterology 120, 1607–1619 (2001).
Reid, B., Blount, P. & Rabinovitch, P. Biomarkers in Barrett's esophagus: a guideline for clinicians. Gastrointest. Clin. N. Am. (in the press).
Alikhan, M. et al. Variable pathologic interpretation of columnar lined esophagus by general pathologists in community practice. Gastrointest. Endosc. 50, 23–26 (1999).
Ofman, J. J. et al. The quality of care in Barrett's esophagus: endoscopist and pathologist practices. Am. J. Gastroenterol. 96, 876–881 (2001).
Macdonald, C. E., Wicks, A. C. & Playford, R. J. Final results from 10 year cohort of patients undergoing surveillance for Barrett's oesophagus: observational study. BMJ 321, 1252–1255 (2000).
Connor, R. J., Prorok, P. C. & Weed, D. L. The case-control design and the assessment of the efficacy of cancer screening. J. Clin. Epidemiol. 44, 1215–1221 (1991).
Cronin, K. A., Weed, D. L., Connor, R. J. & Prorok, P. C. Case-control studies of cancer screening: theory and practice. J. Natl Cancer Inst. 90, 498–504 (1998).
Weiss, N. S. Case-control studies of the efficacy of screening for cancer: can we earn them some respect. J. Med. Screen. 4, 57–59 (1997). References 32–34 discuss the use of case–control studies for evaluating cancer screening with special attention to avoiding bias.
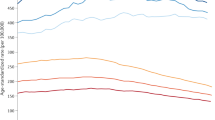
Etzioni, R. et al. Cancer surveillance series: interpreting trends in prostate cancer. Part III: Quantifying the link between population prostate-specific antigen testing and recent declines in prostate cancer mortality. J. Natl Cancer Inst. 91, 1033–1039 (1999).
Feuer, E. J., Mariotto, A. & Merrill, R. Modeling the impact of the decline in distant stage disease on prostate carcinoma mortality rates. Cancer 95, 870–880 (2002).
Schatzkin, A. & Gail, M. The promise and peril of surrogate end points in cancer research. Nature Rev. Cancer 2, 19–27 (2002).
Hirsch, F. R. et al. IV international conference on prevention and early detection of lung cancer, Reykjavik, Iceland, August 9–12, 2001. Lung Cancer 37, 325–344 (2002).
Friedman, D. R. & Dubin, N. Case-control evaluation of breast cancer screening efficacy. Am. J. Epidemiol. 133, 974–984 (1991).
Newcomb, P. A. et al. Breast self-examination in relation to the occurrence of advanced breast cancer. J. Natl Cancer Inst. 83, 260–265 (1991).
Hurley, S. F. & Kaldor, J. M. The benefits and risks of mammographic screening for breast cancer. Epidemiol. Rev. 14, 101–130 (1992).
Palli, D. et al. A case-control study of the efficacy of a non-randomized breast cancer screening program in Florence (Italy). Int. J. Cancer 38, 501–504 (1986).
Selby, J. V., Friedman, G. D., Quesenberry, C. P. Jr & Weiss, N. S. A case-control study of screening sigmoidoscopy and mortality from colorectal cancer. N. Engl. J. Med. 326, 653–657 (1992).
Beemsterboer, P. M. et al. Prostate specific antigen testing and digital rectal examination before and during a randomized trial of screening for prostate cancer: European randomized study of screening for prostate cancer, Rotterdam. J. Urol. 164, 1216–1220 (2000).
Gohagan, J. K., Prorok, P. C., Hayes, R. B. & Kramer, B. S. The Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial of the National Cancer Institute: history, organization, and status. Control Clin. Trials 21, 251S–272S (2000).
Bartsch, G. et al. Prostate cancer mortality after introduction of prostate-specific antigen mass screening in the Federal State of Tyrol, Austria. Urology 58, 417–424 (2001).
Lu–Yao, G. et al. Natural experiment examining impact of aggressive screening and treatment on prostate cancer mortality in two fixed cohorts from Seattle area and Connecticut. BMJ 325, 740 (2002).
Oliver, S. E., May, M. T. & Gunnell, D. International trends in prostate-cancer mortality in the 'PSA ERA'. Int. J. Cancer 92, 893–898 (2001). References 46–48 are examples of recent population studies of PSA screening.
Harris, R. & Lohr, K. N. Screening for prostate cancer: an update of the evidence for the US Preventive Services Task Force. Ann. Intern. Med. 137, 917–929 (2002).
Brown, M. L. & Fintor, L. Cost-effectiveness of breast cancer screening: preliminary results of a systematic review of the literature. Breast Cancer Res. Treat. 25, 113–118 (1993). Explores the reasons for variability in estimates of the cost-effectiveness of screening interventions.
Rosenquist, C. J. & Lindfors, K. K. Screening mammography beginning at age 40 years: a reappraisal of cost-effectiveness. Cancer 82, 2235–2240 (1998).
Salzmann, P., Kerlikowske, K. & Phillips, K. Cost-effectiveness of extending screening mammography guidelines to include women 40 to 49 years of age. Ann. Intern. Med. 127, 955–965 (1997). References 51 and 52 show how different results of cost-effectiveness studies of the same intervention can be.
Pignone, M., Saha, S., Hoerger, T. & Mandelblatt, J. Cost-effectiveness analyses of colorectal cancer screening: a systematic review for the US Preventive Services Task Force. Ann. Intern. Med. 137, 96–104 (2002).
van der Maas, P. J. et al. The cost-effectiveness of breast cancer screening. Int. J. Cancer 43, 1055–1060 (1989).
US Congress Office of Technology Assessment. Costs and Effectiveness of Prostate Cancer Screening in Elderly Men (US Government Printing Office, Washington DC, 1995).
Etzioni, R., Cha, R. & Cowen, M. E. Serial prostate specific antigen screening for prostate cancer: a computer model evaluates competing strategies. J. Urol. 162, 741–748 (1999).
Ross, K. S., Carter, H. B., Pearson, J. D. & Guess, H. A. Comparative efficiency of prostate-specific antigen screening strategies for prostate cancer detection. JAMA 284, 1399–1405 (2000).
Boyes, D. A. The value of a pap smear program and suggestions for its implementation. Cancer 48, 613–621 (1981).
Christopherson, W. M., Parker, J. E. & Mendez, W. M. Cervix cancer death rates and mass cytologic screening. Cancer 26, 808–811 (1970).
Schiffman, M. H., Brinton, L. A., Devesa, S. S. & Fraumeni, J. F. Jr in Cancer Epidemiology and Prevention 2nd edn (eds Schottenfeld, D. & Fraumeni, J. F. Jr) 1090–1116 (Oxford Univ. Press, New York, 1996).
Parkin, D. M., Pisani, P. & Ferlay, J. Estimates of the worldwide frequency of eighteen major cancers in 1985. Int. J. Cancer 54, 594–606 (1993).
Cuzick, J. Time to consider HPV testing in cervical screening. Ann. Oncol. 12, 1511–1514 (2001).
Stoler, M. H. HPV for cervical cancer screening: is the era of the molecular pap smear upon us? J. Histochem. Cytochem. 49, 1197–1198 (2001).
Cuzick, J. et al. Human papillomavirus testing in primary cervical screening. Lancet 345, 1533–1536 (1995).
Kulasingam, S. L. et al. Evaluation of human papillomavirus testing in primary screening for cervical abnormalities: comparison of sensitivity, specificity, and frequency of referral. JAMA 288, 1749–1757 (2002).
Ratnam, S., Franco, E. L. & Ferenczy, A. Human papillomavirus testing for primary screening of cervical cancer precursors. Cancer Epidemiol. Biomarkers Prev. 9, 945–951 (2000).
Woolas, R. P. et al. Combinations of multiple serum markers are superior to individual assays for discriminating malignant from benign pelvic masses. Gynecol. Oncol. 59, 111–116 (1995).
Woolas, R. P. et al. Elevation of multiple serum markers in patients with stage I ovarian cancer. J. Natl Cancer Instit. 85, 1748–1751 (1993).
Bates, J. et al. Clinical value of CYFRA 21.1, carcinoembryonic antigen, neurone-specific enolase, tissue polypeptide specific antigen and tissue polypeptide antigen in the diagnosis of lung cancer. Eur. Resp. J. 10, 2535–2538 (1997).
Beduschi, M. C. & Oesterling, J. E. Percent free prostate-specific antigen: the next frontier in prostate-specific antigen testing. Urology 51, 98–109 (1998).
Brawer, M. K. Prostate-specific antigen: current status. CA Cancer J. Clin. 49, 264–281 (1999).
Catalona, W. J. et al. Use of the percentage of free prostate-specific antigen to enhance differentiation of prostate cancer from benign prostatic disease: a prospective multicenter clinical trial. JAMA 279, 1542–1547 (1998).
Gann, P. H., Ma, J., Catalona, W. J. & Stampfer, M. J. Strategies combining total and percent free prostate specific antigen for detecting prostate cancer: a prospective evaluation. J. Urol. 167, 2427–2434 (2002).
Zhang, Z. et al. Combination of multiple serum markers using an artificial neural network to improve specificity in discriminating malignant from benign pelvic masses. Gynecol. Oncol. 73, 56–61 (1999).
McIntosh, M. W. & Pepe, M. Combining several screening tests: optimality of the risk score. Biometrics 58, 657–664 (2002).
Baker, S. G. Identifying combinations of cancer markers for further study as triggers of early intervention. Biometrics 56, 1082–1087 (2000).
Etzioni, R., Kooperberg, C., Pepe, M. S., Smith, R. & Gann, P. H. Combining biomarkers to detect disease with application to prostate cancer. Biostatistics (in the press). References 74–77 present new statistical methods for identifying optimal combinations of biomarkers.
Optenberg, S. A. et al. Development of a decision-making tool to predict risk of prostate cancer: the Cancer of the Prostate Risk Index (CAPRI) test. Urology 50, 665–672 (1997).
Urban, N., Drescher, C., Clarke, L. & Kiviat, N. in Ovarian Cancer 5 (eds Sharp, F., Blackett, T., Berek, J. & Bast, R.) 217–229 (Isis Medical Media Ltd., Oxford, 1998).
Urban, N. et al. in Ovarian Cancer (ed. Jacobs, I.) 199–208 (Oxford Univ. Press, 2002).
Urban, N. Screening for ovarian cancer. BMJ 319, 1317–1318 (1999).
American Cancer Society. Costs of Cancer, 2002 [online], (cited 25 Jan 2003), <http://www.cancer.org/docroot/MIT/content/MIT_3_2X_Costs_of_Cancer.asp> (2002).
Gold, M. E., Siegel, J. E., Russell, L. B. & Weintstein, M. C. Cost–Effectiveness in Health and Medicine (Oxford Univ. Press, New York, 1996).
US Congress Office of Technology Assessment. Breast Cancer Screening for Medicare Beneficiaries; Effectiveness, Costs to Medicare and Medical Resources Required (US Government Prinitng Office, Washington DC, 1987).
Shibata, A. & Whittemore, A. S. Re: Prostate cancer incidence and mortality in the United States and the United Kingdom. J. Natl Cancer Inst. 93, 1109–1110 (2001).
Crocetti, E., Ciatto, S. & Zappa, M. Prostate cancer: different incidence but not mortality trends within two areas of Tuscany, Italy. J. Natl Cancer Inst. 93, 876–877 (2001).
Acknowledgements
Supported in part by cooperative agreement from the National Cancer Institute. We thank R. Smith of the American Cancer Society and the anonymous referees for helpful comments on an earlier version of this article.
Author information
Authors and Affiliations
Corresponding author
Related links
Related links
DATABASES
Cancer.gov
LocusLink
Glossary
- PROTEOMICS
-
The characterization and quantification of proteins and protein systems. Proteomics methods allow for the comparison of patterns of proteins isolated from bodily fluids or cells, in normal versus diseased subjects.
- FALSE-NEGATIVE RATE
-
The proportion of diseased subjects that test negative.
- FALSE-POSITIVE RATE
-
The proportion of non-diseased (healthy) subjects that test positive.
- OVERDIAGNOSIS
-
The detection by screening of disease that, in the absence of screening, would not have been diagnosed within the lifetime of the patient.
- SIGMOIDOSCOPY
-
A test that is used to detect colo-rectal cancer. A thin, flexible, hollow tube (sigmoidoscope) is inserted into the rectum for imaging of the lower part of the colon and rectum.
- COLONOSCOPY
-
Similar to sigmoidoscopy, but examines the entire length of the colon.
- SENSITIVITY
-
The proportion of diseased subjects that test positive.
- SPECIFICITY
-
The proportion of non-diseased (healthy) subjects that test negative.
- PROSTATE-SPECIFIC ANTIGEN
-
(PSA). A glycoprotein that is produced primarily by the epithelial cells of the prostate gland. PSA levels in serum are generally low but increase in most patients with prostate cancer.
- GENE-EXPRESSION ANALYSIS
-
The measurement of the expression of thousands of genes simultanously.
- PAPANICOLAU (PAP) SMEAR
-
An exfoliative cytological staining procedure that can detect premalignant and malignant changes in the cervical epithelium and that is named after its founder.
- HUMAN PAPILLOMAVIRUS
-
(HPV). A virus that causes genital warts. It has also been shown to cause cervical cancer.
- T-TEST
-
A statistical procedure for comparing measurements in two groups or samples. The result of a t-test provides an assessment of the difference between the average value in each sample relative to the variability in the two samples.
- RECEIVER-OPERATING CHARACTERISTIC (ROC) CURVE
-
A graph of the false-negative rate versus the false-positive rate corresponding to a biomarker-based test, as the threshold biomarker level (or cutoff) for declaring the test positive varies.
- CLUSTER ANALYSIS
-
A technique for grouping a collection of objects into subsets or clusters such that those within each cluster are more closely related to one another than are objects assigned to different clusters.
- SUPPORT VECTOR MACHINES
-
A technique for separating data points into classes. Support vector machines derive nonlinear boundaries to optimally separate clouds of points.
- ELISA
-
(Enzyme-linked immunosorbent assay). A widely used technique for determining the presence or amount of protein in a biological sample, using an enzyme that bonds to an antibody or antigen and causes a colour change.
- SELDI-TOF
-
(Surface-enhanced laser desorption — time of flight). A method for profiling a population of proteins in a sample according to the size and net electrical charge of the individual proteins. The position of an individual protein in the spectrum produced corresponds to its 'time of flight' because the small proteins fly faster and the large proteins fly more slowly.
Rights and permissions
About this article
Cite this article
Etzioni, R., Urban, N., Ramsey, S. et al. The case for early detection. Nat Rev Cancer 3, 243–252 (2003). https://doi.org/10.1038/nrc1041
Issue Date:
DOI: https://doi.org/10.1038/nrc1041
This article is cited by
-
Submonolayer biolasers for ultrasensitive biomarker detection
Light: Science & Applications (2023)
-
Detection and analysis of chiral molecules as disease biomarkers
Nature Reviews Chemistry (2023)
-
A non-invasive method for concurrent detection of multiple early-stage cancers in women
Scientific Reports (2023)
-
Aptamer-based gold nanoparticle aggregates for ultrasensitive amplification-free detection of PSMA
Scientific Reports (2023)
-
Molecular imaging analysis in cancer using deep learning: a review
Research on Biomedical Engineering (2023)