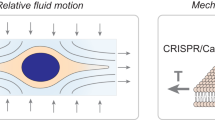
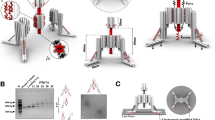
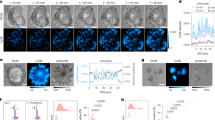
Abstract
Spatiotemporal interrogation of signal transduction at the single-cell level is necessary to answer a host of important biological questions. This protocol describes a nanotechnology-based single-cell and single-molecule perturbation tool, termed mechanogenetics, that enables precise spatial and mechanical control over genetically encoded cell-surface receptors in live cells. The key components of this tool are a magnetoplasmonic nanoparticle (MPN) actuator that delivers defined spatial and mechanical cues to receptors through target-specific one-to-one engagement and a micromagnetic tweezers (μMT) that remotely controls the magnitude of force exerted on a single MPN. In our approach, a SNAP-tagged cell-surface receptor of interest is conjugated with a single-stranded DNA oligonucleotide, which hybridizes to its complementary oligonucleotide on the MPN. This protocol consists of four major stages: (i) chemical synthesis of MPNs, (ii) conjugation with DNA and purification of monovalent MPNs, (iii) modular targeting of MPNs to cell-surface receptors, and (iv) control of spatial and mechanical properties of targeted mechanosensitive receptors in live cells by adjusting the μMT-to-MPN distance. Using benzylguanine (BG)-functionalized MPNs and model cell lines expressing either SNAP-tagged Notch or vascular endothelial cadherin (VE-cadherin), we provide stepwise instructions for mechanogenetic control of receptor clustering and for mechanical receptor activation. The ability of this method to differentially control spatial and mechanical inputs to targeted receptors makes it particularly useful for interrogating the differential contributions of each individual cue to cell signaling. The entire procedure takes up to 1 week.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
References
Banghart, M., Borges, K., Isacoff, E., Trauner, D. & Kramer, R.H. Light-activated ion channels for remote control of neuronal firing. Nat. Neurosci. 7, 1381–1386 (2004).
Deisseroth, K. Optogenetics. Nat. Methods 8, 26–29 (2011).
Miesenbock, G. The optogenetic catechism. Science 326, 395–399 (2009).
Toettcher, J.E., Voigt, C.A., Weiner, O.D. & Lim, W.A. The promise of optogenetics in cell biology: interrogating molecular circuits in space and time. Nat. Methods 8, 35–38 (2011).
Wu, Y.I. et al. A genetically encoded photoactivatable Rac controls the motility of living cells. Nature 461, 104–108 (2009).
Mannix, R.J. et al. Nanomagnetic actuation of receptor-mediated signal transduction. Nat. Nanotechnol. 3, 36–40 (2008).
Lee, J.H. et al. Artificial control of cell signaling and growth by magnetic nanoparticles. Angew. Chem. Int. Ed. Engl. 49, 5698–5702 (2010).
Cho, M.H. et al. A magnetic switch for the control of cell death signalling in in vitro and in vivo systems. Nat. Mater. 11, 1038–1043 (2012).
Etoc, F. et al. Subcellular control of Rac-GTPase signalling by magnetogenetic manipulation inside living cells. Nat. Nanotechnol. 8, 193–198 (2013).
Hoffmann, C. et al. Spatiotemporal control of microtubule nucleation and assembly using magnetic nanoparticles. Nat. Nanotechnol. 8, 199–205 (2013).
Liu, Z. et al. Nanoscale optomechanical actuators for controlling mechanotransduction in living cells. Nat. Methods 13, 143–146 (2016).
Seo, D. et al. A mechanogenetic toolkit for interrogating cell signaling in space and time. Cell 165, 1507–1518 (2016).
Huang, H., Delikanli, S., Zeng, H., Ferkey, D.M. & Pralle, A. Remote control of ion channels and neurons through magnetic-field heating of nanoparticles. Nat. Nanotechnol. 5, 602–606 (2010).
Chen, R., Romero, G., Christiansen, M.G., Mohr, A. & Anikeeva, P. Wireless magnetothermal deep brain stimulation. Science 347, 1477–1480 (2015).
Stanley, S.A. et al. Radio-wave heating of iron oxide nanoparticles can regulate plasma glucose in mice. Science 336, 604–608 (2012).
Stanley, S.A., Sauer, J., Kane, R.S., Dordick, J.S. & Friedman, J.M. Remote regulation of glucose homeostasis in mice using genetically encoded nanoparticles. Nat. Med. 21, 92–98 (2015).
Carvalho de Souza, J.L. et al. Photosensitivity of neurons enabled by cell-targeted gold nanoparticles. Neuron 86, 207–217 (2015).
Meister, M. Physical limits to magnetogenetics. Elife 5, e17210 (2016).
Howarth, M. et al. Monovalent, reduced-size quantum dots for imaging receptors on living cells. Nat. Methods 5, 397–399 (2008).
Farlow, J. et al. Formation of targeted monovalent quantum dots by steric exclusion. Nat. Methods 10, 1203–1205 (2013).
Banerjee, D., Liu, A.P., Voss, N.R., Schmid, S.L. & Finn, M.G. Multivalent display and receptor-mediated endocytosis of transferrin on virus-like particles. Chembiochem 11, 1273–1279 (2010).
Dustin, M.L. & Zhu, C. T cells like a firm molecular handshake. Proc. Natl. Acad. Sci. USA 103, 4335–4336 (2006).
Das, D.K. et al. Force-dependent transition in the T-cell receptor β-subunit allosterically regulates peptide discrimination and pMHC bond lifetime. Proc. Natl. Acad. Sci. USA 112, 1517–1522 (2015).
Dufrene, Y.F. et al. Five challenges to bringing single-molecule force spectroscopy into living cells. Nat. Methods 8, 123–127 (2011).
Neuman, K.C. & Nagy, A. Single-molecule force spectroscopy: optical tweezers, magnetic tweezers and atomic force microscopy. Nat. Methods 5, 491–505 (2008).
Jiang, W., Kim, B.Y., Rutka, J.T. & Chan, W.C. Nanoparticle-mediated cellular response is size-dependent. Nat. Nanotechnol. 3, 145–150 (2008).
Xu, S., Olenyuk, B.Z., Okamoto, C.T. & Hamm-Alvarez, S.F. Targeting receptor-mediated endocytotic pathways with nanoparticles: rationale and advances. Adv. Drug Deliv. Rev. 65, 121–138 (2013).
Levskaya, A., Weiner, O.D., Lim, W.A. & Voigt, C.A. Spatiotemporal control of cell signalling using a light-switchable protein interaction. Nature 461, 997–1001 (2009).
Shete, H.K., Prabhu, R.H. & Patravale, V.B. Endosomal escape: a bottleneck in intracellular delivery. J. Nanosci. Nanotechnol. 14, 460–474 (2014).
Martens, T.F., Remaut, K., Demeester, J., Smedt, S.C.D. & Braeckmans, K. Intracellular delivery of nanomaterials: how to catch endosomal escape in the act. Nano Today 9, 344–364 (2014).
Tseng, P., Judy, J.W. & Di Carlo, D. Magnetic nanoparticle-mediated massively parallel mechanical modulation of single-cell behavior. Nat. Methods 9, 1113–1119 (2012).
Wheeler, M.A. et al. Genetically targeted magnetic control of the nervous system. Nat. Neurosci. 19, 756–761 (2016).
Tseng, P. et al. Flexible and stretchable micromagnet arrays for tunable biointerfacing. Adv. Mater. 27, 1083–1089 (2015).
Farrell, D., Majetich, S.A. & Wilcoxon, J.P. Preparation and characterization of monodisperse Fe nanoparticles. J. Phys. Chem. B 107, 11022–11030 (2003).
Zhang, S. et al. Halide ion-mediated growth of single crystalline Fe nanoparticles. Nanoscale 6, 4852–4856 (2014).
Zalich, M.A., Baranauskas, V.V., Riffle, J.S., Saunders, M. & Pierre, T.G.S. Structural and magnetic properties of oxidatively stable cobalt nanoparticles encapsulated in graphite shells. Chem. Mater. 18, 2648–2655 (2006).
Pankhurst, Q.A., Connolly, J., Jones, S.K. & Dobson, J. Applications of magnetic nanoparticles in biomedicine. J. Phys. D 36, 167–181 (2003).
Matthews, B.D., LaVan, D.A., Overby, D.R., Karavitis, J. & Ingber, D.E. Electromagnetic needles with submicron pole tip radii for nanomanipulation of biomolecules and living cells. Appl. Phys. Lett. 85, 2968–2970 (2004).
Yan, Z. et al. Drosophila NOMPC is a mechanotransduction channel subunit for gentle-touch sensation. Nature 493, 221–225 (2013).
Coste, B. et al. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science 330, 55–60 (2010).
Kim, S.E., Coste, B., Chadha, A., Cook, B. & Patapoutian, A. The role of Drosophila Piezo in mechanical nociception. Nature 483, 209–212 (2012).
van der Merwe, P.A. The TCR triggering puzzle. Immunity 14, 665–668 (2001).
Wichert, G.v. et al. RPTP-α acts as a transducer of mechanical force on αv/β3-integrin-cytoskeleton linkages. J. Cell Biol. 161, 143–153 (2003).
Huang, R.B. & Eniola-Adefeso, O. PLoS One 7, e31874 (2012).
Marshall, B.T. et al. Direct observation of catch bonds involving cell-adhesion molecules. Nature 423, 190–193 (2003).
Tzima, E. et al. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature 437, 426–431 (2005).
Chen, Y. et al. Fluorescence biomembrane force probe: concurrent quantitation of receptor-ligand kinetics and binding-induced intracellular signaling on a single cell. J. Vis. Exp. 102, e52975 (2015).
Luca, V.C. et al. Notch-Jagged complex structure implicates a catch bond in tuning ligand sensitivity. Science 355, 1320–1324 (2017).
Jang, J.T. et al. Critical enhancements of MRI contrast and hyperthermic effects by dopant-controlled magnetic nanoparticles. Angew. Chem. Int. Ed. Engl. 48, 1234–1238 (2009).
Prodan, E., Radloff, C., Halas, N.J. & Nordlander, P. A hybridization model for the plasmon response of complex nanostructures. Science 302, 419–422 (2003).
Jain, P.K., Lee, K.S., El-Sayed, I.H. & El-Sayed, M.A. Calculated absorption and scattering properties of gold nanoparticles of different size, shape, and composition: applications in biological imaging and biomedicine. J. Phys. Chem. B 110, 7238–7248 (2006).
Cullity, B.D. Introduction to Magnetic Materials (Addison-Wesley Pub., 1972).
Bercoff, P.G. & Bertorello, H.R. Exchange constants and transfer integrals of spinel ferrites. J. Magn. Magn. Mater. 169, 314–322 (1997).
Schiessl, W. et al. Magnetic properties of the ZnFe2O4 spinel. Phys. Rev. B Condens. Matter 53, 9143–9152 (1996).
Yi, D.K., Lee, S.S., Papaefthymiou, G.C. & Ying, J.Y. Nanoparticle architectures templated by SiO2/Fe2O3 nanocomposites. Chem. Mater. 18, 614–619 (2006).
Oldenburg, S.J., Averitt, R.D., Westcott, S.L. & Halas, N.J. Nanoengineering of optical resonances. Chem. Phys. Lett. 288, 243–247 (1998).
Brinson, B.E. et al. Nanoshells made easy: improving Au layer growth on nanoparticle surfaces. Langmuir 24, 14166–14171 (2008).
Sarah, L.W., Oldenburg, S.J., Lee, T.R. & Halas, N.J. Formation and adsorption of clusters of gold nanoparticles onto functionalized silica nanoparticle surfaces. Langmuir 14, 5396–5401 (1998).
Claridge, S.A., Liang, H.W., Basu, S.R., Frechet, J.M. & Alivisatos, A.P. Isolation of discrete nanoparticle-DNA conjugates for plasmonic applications. Nano Lett. 8, 1202–1206 (2008).
Seo, D., Lee, H., Lee, J.-u., Haas, T.J. & Jun, Y.-w. Monovalent plasmonic nanoparticles for biological applications. Proc. SPIE 9722, 97220I–97221 (2016).
Batchelor, G.K. An Introduction to Fluid Dynamics (Cambridge University Press, 1967).
Rogers, H.B., Anani, T., Choi, Y.S., Beyers, R.J. & David, A.E. Exploiting size-dependent drag and magnetic forces for size-specific separation of magnetic nanoparticles. Int. J. Mol. Sci. 16, 20001–20019 (2015).
Wang, X. & Ha, T. Defining single molecular forces required to activate integrin and notch signaling. Science 340, 991–994 (2013).
Ho, D. et al. Force-driven separation of short double-stranded DNA. Biophys. J. 97, 3158–3167 (2009).
Kuhner, F., Morfill, J., Neher, R.A., Blank, K. & Gaub, H.E. Force-induced DNA slippage. Biophys. J. 92, 2491–2497 (2007).
Cocco, S., Monasson, R. & Marko, J.F. Force and kinetic barriers to unzipping of the DNA double helix. Proc. Natl. Acad. Sci. USA 98, 8608–8613 (2001).
Jain, P.K. & El-Sayed, M.A. Universal scaling of plasmon coupling in metal nanostructures: extension from particle pairs to nanoshells. Nano Lett. 7, 2854–2858 (2007).
Wang, Y. et al. Visualizing the mechanical activation of Src. Nature 434, 1040–1045 (2005).
Acknowledgements
We thank S. Blacklow (Harvard University) for the generous gift of Notch plasmids. This work was supported by IBS-R026-D1 from IBS (Y.J. and J.C.), HI08C2149 from Korea Healthcare Technology R&D Project (J.C.), 2017R1C1B2010945 from a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIP, D.S.), 1R01GM112081-01 from the National Institute of General Medical Science (NIGMS) and the National Institute of Health (NIH) (Y.J.), the UCSF Program for Breakthrough Biomedical Research–Sandler Foundation (Y.J.), 1R21HL123329-01 from the National Heart, Lung, and Blood Institute and NIH (Y.J.), DP2 HD080351-01 from the NIH common fund (Z.J.G.) and P50 GM081879 from the NIGMS UCSF Center for Synthetic and Systems Biology (Z.J.G.).
Author information
Authors and Affiliations
Contributions
J.K., D.S., Y.J. and J.C. conceived and designed the project; D.S., J.K., J.L., Y.L., D.K., Y.J. and J.C. contributed to nanoparticle synthesis; D.S., K.M.S., Z.J.G. and Y.J. designed the biological experiments; J.K., D.S., J.L., K.M.S., Y.L. and D.K. performed experiments and analyzed the data; and J.K., D.S., J.L., Y.J. and J.C. wrote the manuscript. All authors discussed and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Functional group-dependent chemical stability of M-SiO2(Au2nm)n.
Reaction of Au2nm seeds with 20 nm APTMS or AEAPTMS functionalized M-SiO2 yielded 161±11 and 165±10 Au seeds per M-SiO2, respectively. The chemical stability of the respective conjugates was examined by incubating them in 1 mM Tris-HCl buffer (pH 8.0) for 48 hr. Significant portion of Au seeds were detached from M-SiO2(Au2nm)n (Au seeds per M-SiO2 after 48hr incubation: 98.1±17) produced by the APTMS conjugation chemistry, whereas negligible changes were observed for the AEAPTMS case (n = 161±15). Scale bar, 10 nm.
Supplementary Figure 2 Synthesis of MPNs having a 30 nm iron oxide magnetic core.
(a) TEM images of 30 nm iron oxide magnetic nanoparticles, (b) silica-coated magnetic nanoparticles (silica thickness, 4 nm), and (c) MPNs (diameter, 55 ± 6.7 nm). 30 nm iron oxide nanoparticles are used in step 14. Scale bar, 50 nm.
Supplementary Figure 3 Time dependent changes in absorbance of the gold growth solution.
(a) UV-Vis absorption spectra and (b) changes in absorbance at 290 nm of the gold growth solution as a function of time.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–3 (PDF 392 kb)
Rights and permissions
About this article
Cite this article
Kim, Jw., Seo, D., Lee, Ju. et al. Single-cell mechanogenetics using monovalent magnetoplasmonic nanoparticles. Nat Protoc 12, 1871–1889 (2017). https://doi.org/10.1038/nprot.2017.071
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2017.071
This article is cited by
-
Magneto-acoustic protein nanostructures for non-invasive imaging of tissue mechanics in vivo
Nature Materials (2024)
-
A magnetically powered nanomachine with a DNA clutch
Nature Nanotechnology (2024)
-
Modulating cell signalling in vivo with magnetic nanotransducers
Nature Reviews Methods Primers (2022)
-
Adherens junctions organize size-selective proteolytic hotspots critical for Notch signalling
Nature Cell Biology (2022)
-
Non-contact long-range magnetic stimulation of mechanosensitive ion channels in freely moving animals
Nature Materials (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.