Abstract
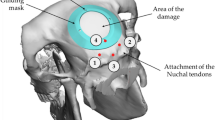
Translational biomaterials targeted toward the regeneration of large bone defects in the mandible require a preclinical model that accurately recapitulates the regenerative challenges present in humans. Computational modeling and in vitro assays do not fully replicate the in vivo environment. Consequently, in vivo models can have specific applications such as those of the mandibular angle defect, which is used to investigate bone regeneration in a nonload-bearing area, and the inferior border mandibular defect, which is a model for composite bone and nerve regeneration, with both models avoiding involvement of soft tissue or teeth. In this protocol, we describe a reproducible load-bearing critical-size composite tissue defect comprising loss of soft tissue, bone and tooth in the mandible of a rabbit. We have previously used this procedure to investigate bone regeneration, vascularization and infection prevention in response to new biomaterial formulations for craniofacial tissue engineering applications. This surgical approach can be adapted to investigate models such as that of regeneration in the context of osteoporosis or irradiation. The procedure can be performed by researchers with basic surgical skills such as dissection and suturing. The procedure takes 1.5–2 h, with ∼2 h of immediate postoperative care, and animals should be monitored daily for the remainder of the study. For bone tissue engineering applications, tissue collection typically occurs 12 weeks after surgery. In this protocol, we will present the necessary steps to ensure reproducibility; tips to minimize complications during and after surgery; and analytical techniques for assessing soft tissue, bone and vessel regeneration by gross evaluation, microcomputed tomography (microCT) and histology.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout











Similar content being viewed by others
References
Smith, B.T., Shum, J., Wong, M., Mikos, A.G. & Young, S. Bone tissue engineering challenges in oral & maxillofacial surgery. Adv. Exp. Med. Biol. 881, 57–78 (2015).
Schmitz, J.P. & Hollinger, J.O. The critical size defect as an experimental-model for craniomandibulofacial nonunions. Clin. Orthop. Relat. Res. 205, 299–308 (1986).
Takagi, K. & Urist, M.R. The reaction of the dura to bone morphogenetic protein (Bmp) in repair of skull defects. Ann. Surg. 196, 100–109 (1982).
Young, S. et al. Development and characterization of a rabbit alveolar bone nonhealing defect model. J. Biomed. Mater. Res. A 86A, 182–194 (2008).
Spicer, P.P. et al. Evaluation of bone regeneration using the rat critical size calvarial defect. Nat. Protoc. 7, 1918–1929 (2012).
Cooper, G.M. et al. Testing the critical size in calvarial bone defects: revisiting the concept of a critical-size defect. Plast. Reconstr. Surg. 125, 1685–1692 (2010).
Kasper, F.K., Tanahashi, K., Fisher, J.P. & Mikos, A.G. Synthesis of poly(propylene fumarate). Nat. Protoc. 4, 518–525 (2009).
Kinard, L.A., Kasper, F.K. & Mikos, A.G. Synthesis of oligo(poly(ethylene glycol) fumarate). Nat. Protoc. 7, 1219–1227 (2012).
Watson, B.M. et al. Biodegradable, phosphate-containing, dual-gelling macromers for cellular delivery in bone tissue engineering. Biomaterials 67, 286–296 (2015).
Sanz-Herrera, J.A. & Reina-Romo, E. Cell-biomaterial mechanical interaction in the framework of tissue engineering: insights, computational modeling and perspectives. Int. J. Mol. Sci. 12, 8217–8244 (2011).
Bancroft, G.N., Sikavitsas, V.I. & Mikos, A.G. Design of a flow perfusion bioreactor system for bone tissue-engineering applications. Tissue Eng. 9, 549–554 (2003).
Tatara, A.M., Shah, S.R., Livingston, C.E. & Mikos, A.G. Infected animal models for tissue engineering. Methods 84, 17–24 (2015).
Mankani, M.H., Kuznetsov, S.A., Wolfe, R.M., Marshall, G.W. & Robey, P.G. In vivo bone formation by human bone marrow stromal cells: reconstruction of the mouse calvarium and mandible. Stem Cells 24, 2140–2149 (2006).
Saadeh, P.B. et al. Repair of a critical size defect in the rat mandible using allogenic type I collagen. J. Craniofac. Surg. 12, 573–579 (2001).
van Leeuwen, A.C. et al. Guided bone regeneration in rat mandibular defects using resorbable poly(trimethylene carbonate) barrier membranes. Acta Biomater. 8, 1422–1429 (2012).
Stubinger, S. & Dard, M. The rabbit as experimental model for research in implant dentistry and related tissue regeneration. J. Invest. Surg. 26, 266–282 (2013).
Elsalanty, M.E. et al. Reconstruction of canine mandibular bone defects using a bone transport reconstruction plate. Ann. Plast. Surg. 63, 441–448 (2009).
Nienhuijs, M.E.L. et al. Healing of bone defects in the goat mandible, using COLLOSS (R) E and beta-tricalciumphosphate. J. Biomed. Mater. Res. B 92B, 517–524 (2010).
Zhou, L.B. et al. Reconstruction of curved mandibular angle defects using a new internal transport distraction device: an experiment in goats. Br. J. Oral Maxillofac. Surg. 46, 445–448 (2008).
Ma, J.L., Pan, J.L., Tan, B.S. & Cui, F.Z. Determination of critical size defect of minipig mandible. J. Tissue Eng. Regen. Med. 3, 615–622 (2009).
Sun, Z.Y., Kennedy, K.S., Tee, B.C., Damron, J.B. & Allen, M.J. Establishing a critical-size mandibular defect model in growing pigs: characterization of spontaneous healing. J. Oral Maxillofac. Surg. 72, 1852–1868 (2014).
Tatara, A.M. et al. Autologously generated tissue-engineered bone flaps for reconstruction of large mandibular defects in an ovine model. Tissue Eng. Part A 21, 1520–1528 (2015).
Dard, M. Animal models for experimental surgical research in implant dentistry. in Implant Dentistry Research Guide: Basic, Translational and Experimental Clinical Research. (ed. Ballo, A.) 167–190 (Nova Science Publishers, New York, 2012).
Dard, M. Methods and interpretation of performance studies for dental implants. in Biocompatibility and Performance of Medical Devices (ed. Jean-Pierre Boutrand) 308–344 (Woodhead Publishing, Cambridge, UK, 2012).
Guo, J. et al. Restoration of critical-size defects in the rabbit mandible using porous nanohydroxyapatite-polyamide scaffolds. Tissue Eng. Part A 18, 1239–1252 (2012).
Campillo, V.E., Langonnet, S., Pierrefeu, A. & Chaux-Bodard, A.G. Anatomic and histological study of the rabbit mandible as an experimental model for wound healing and surgical therapies. Lab. Anim. 48, 273–277 (2014).
Miloro, M., Haralson, D.J. & Desa, V. Bone healing in a rabbit mandibular defect using platelet-rich plasma. J. Oral Maxillofac. Surg. 68, 1225–1230 (2010).
Rottgers, S.A. et al. Bone morphogenetic protein 2-mediated mandible reconstruction successfully heals bony defects but inhibits concurrent inferior alveolar nerve grafting: a rabbit experimental model. J. Craniofac. Surg. 25, 2241–2245 (2014).
Kretlow, J.D. et al. Evaluation of soft tissue coverage over porous polymethylmethacrylate space maintainers within nonhealing alveolar bone defects. Tissue Eng. Part C Methods 16, 1427–1438 (2010).
Nguyen, C., Young, S., Kretlow, J.D., Mikos, A.G. & Wong, M. Surface characteristics of biomaterials used for space maintenance in a mandibular defect: a pilot animal study. J. Oral Maxillofac. Surg. 69, 11–18 (2011).
Spicer, P.P. et al. In situ formation of porous space maintainers in a composite tissue defect. J. Biomed. Mater. Res. A 100A, 827–833 (2012).
Petersen, K. et al. Prevention of infections associated with combat-related eye, maxillofacial, and neck injuries. J. Trauma 71, S264–S269 (2011).
Spicer, P.P. et al. Evaluation of antibiotic releasing porous polymethylmethacrylate space maintainers in an infected composite tissue defect model. Acta Biomater. 9, 8832–8839 (2013).
Shah, S.R. et al. Polymer-based local antibiotic delivery for prevention of polymicrobial infection in contaminated mandibular implants. ACS Biomater. Sci. Eng. 2, 558–566 (2016).
Young, S. et al. Microcomputed tomography characterization of neovascularization in bone tissue engineering applications. Tissue Eng. Part B Rev. 14, 295–306 (2008).
Lalani, Z., Wong, M., Brey, E.M., Mikos, A.G. & Duke, P.J. Spatial and temporal localization of transforming growth factor-beta1, bone morphogenetic protein-2, and platelet-derived growth factor-A in healing tooth extraction sockets in a rabbit model. J. Oral Maxillofac. Surg. 61, 1061–1072 (2003).
Wen, B. et al. The osseointegration behavior of titanium-zirconium implants in ovariectomized rabbits. Clin. Oral Implants Res. 25, 819–825 (2014).
Schon, R., Ohno, K., Kudo, M. & Michi, K. Peri-implant tissue reaction in bone irradiated the fifth day after implantation in rabbits: histologic and histomorphometric measurements. Int. J. Oral Maxillofac. Implants 11, 228–238 (1996).
Jegoux, F. et al. Alveolar ridge augmentation in irradiated rabbit mandibles. J. Biomed. Mater. Res. A 93, 1519–1526 (2010).
German, C.L. & Madihally, S.V. Applications of computational modelling and simulation of porous medium in tissue engineering. Computation 4, article 7, http://dx.doi.org/10.3390/computation4010007 (2016).
Collinet-Adler, S., Castro, C.A., Ledonio, C.G., Bechtold, J.E. & Tsukayama, D.T. Acinetobacter baumannii is not associated with osteomyelitis in a rat model: a pilot study. Clin. Orthop. Relat. Res. 469, 274–282 (2011).
Vo, T.N., Ekenseair, A.K., Kasper, F.K. & Mikos, A.G. Synthesis, physicochemical characterization, and cytocompatibility of bioresorbable, dual-gelling injectable hydrogels. Biomacromolecules 15, 132–142 (2014).
Sarhaddi, D. et al. Validation of histologic bone analysis following microfil vessel perfusion. J. Histotechnol. 35, 180–183 (2012).
Fischer, E.R., Hansen, B.T., Nair, V., Hoyt, F.H. & Dorward, D.W. Scanning electron microscopy. Curr. Protoc. Microbiol. Chapter 2 Unit 2B 2, (2012).
Graham, L. & Orenstein, J.M. Processing tissue and cells for transmission electron microscopy in diagnostic pathology and research. Nat. Protoc. 2, 2439–2450 (2007).
Webster, J.D., Miller, M.A., Dusold, D. & Ramos-Vara, J. Effects of prolonged formalin fixation on diagnostic immunohistochemistry in domestic animals. J. Histochem. Cytochem. 57, 753–761 (2009).
Kallai, I. et al. Microcomputed tomography-based structural analysis of various bone tissue regeneration models. Nat. Protoc. 6, 105–110 (2011).
Meganck, J.A., Kozloff, K.M., Thornton, M.M., Broski, S.M. & Goldstein, S.A. Beam hardening artifacts in micro-computed tomography scanning can be reduced by X-ray beam filtration and the resulting images can be used to accurately measure BMD. Bone 45, 1104–1116 (2009).
An, Y.H. & Martin, K.L. Handbook of Histology Methods for Bone and Cartilage (Humana Press, New York, 2003).
Patel, Z.S. et al. Dual delivery of an angiogenic and an osteogenic growth factor for bone regeneration in a critical size defect model. Bone 43, 931–940 (2008).
Taschereau, R., Silverman, R.W. & Chatziioannou, A.F. Dual-energy attenuation coefficient decomposition with differential filtration and application to a microCT scanner. Phys. Med. Biol. 55, 1141–1155 (2010).
Acknowledgements
A.G.M. acknowledges support toward the development of biomaterials for tissue engineering applications by the National Institutes of Health (grant R01 AR068073). A.G.M. and M.E.W. acknowledge support toward the development of materials and techniques through the Armed Forces Institute of Regenerative Medicine (award no. W81XWH-14-2-0004). S.R.S. acknowledges support from a Ruth L. Kirschstein Fellowship from the National Institutes of Health (F30 AR067606). We acknowledge A. Tatara, J. Lam and S. Lu for their assistance with photography, P. Spicer for sharing his experience with the model and S. Frazier for her assistance in providing details of analgesia and intraoperative monitoring.
Author information
Authors and Affiliations
Contributions
S.R.S. wrote the protocol and developed adaptations to the protocol; S.Y., M.E.W. and A.G.M. developed the protocol; J.L.G. developed the veterinary care plan for the protocol; J.A.J. assisted with the development of analytical protocols.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Notes 1–4 and Supplementary Tables 1 and 2 (PDF 586 kb)
Rights and permissions
About this article
Cite this article
Shah, S., Young, S., Goldman, J. et al. A composite critical-size rabbit mandibular defect for evaluation of craniofacial tissue regeneration. Nat Protoc 11, 1989–2009 (2016). https://doi.org/10.1038/nprot.2016.122
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2016.122
This article is cited by
-
Effect of systemic atorvastatin on bone regeneration in critical-sized defects in hyperlipidemia: an experimental study
International Journal of Implant Dentistry (2023)
-
Materials design for bone-tissue engineering
Nature Reviews Materials (2020)
-
Efficacy of three-dimensionally printed polycaprolactone/beta tricalcium phosphate scaffold on mandibular reconstruction
Scientific Reports (2020)
-
Pre-vascularization in fibrin Gel/PLGA microsphere scaffolds designed for bone regeneration
NPG Asia Materials (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.