Abstract
The cannabinoid receptor type 1 (CB1) and the central nucleus of the amygdala (CeA) are both known to have crucial roles in the processing of fear and anxiety, whereby they appear to be especially involved in the control of fear states. However, in contrast to many other brain regions including the cortical subregions of the amygdala, the existence of CB1 in the CeA remains enigmatic. In this study we show that CB1 is expressed in the CeA of mice and that CB1 in the CeA mediates short-term synaptic plasticity, namely depolarization-induced suppression of excitation (DSE) and inhibition (DSI). Moreover, the CB1 antagonist AM251 increased both excitatory and inhibitory postsynaptic responses in CeA neurons. Local application of AM251 in the CeA in vivo resulted in an acutely increased fear response in an auditory fear conditioning paradigm. Upon application of AM251 in the basolateral nucleus of the amygdala (BLA) in an otherwise identical protocol, no such acute behavioral effects were detected, but CB1 blockade resulted in increased fear responses during tone exposures on the subsequent days. Moreover, we observed that the efficacy of DSE and DSI in the CeA was increased on the day following fear conditioning, indicating that a single tone-shock pairing resulted in changes in endocannabinoid signaling in the CeA. Taken together, our data show the existence of CB1 proteins in the CeA, and their critical role for ensuring short-term adaptation of responses to fearful events, thereby suggesting a potential therapeutic target to accompany habituation-based therapies of post-traumatic symptoms.
Similar content being viewed by others
INTRODUCTION
The therapy of anxiety disorders benefits strongly from a profound understanding of the biological mechanisms that control the intensity and temporal course of fear reactions. Among those mechanisms, accumulating evidence suggests that the endocannabinoid system may have a major role because of its anxiolytic-like properties (Navarro et al, 1997; Patel and Hillard, 2006; for review see Viveros et al, 2005; Wotjak, 2005) and its involvement in the extinction of aversive memories (Marsicano et al, 2002; Suzuki et al, 2004; Chhatwal et al, 2005; Kamprath et al, 2006; for review see Chhatwal and Ressler, 2007). Endocannabinoids are fatty acid derivates that are synthesized and released on demand, for example, upon postsynaptic depolarization or activation of metabotropic glutamate receptors, and bind to presynaptically located G-protein-coupled receptors, the cannabinoid receptors. The cannabinoid receptor type 1 (CB1) ranks among the most abundant G-protein-coupled receptors in the mammalian brain and its activation results in the reduction of transmitter release (eg, GABA, glutamate, or serotonin) from the respective presynapse (for review, see Wilson and Nicoll, 2002; Freund et al, 2003; Di Marzo, 2009). Recent data obtained in humans show that certain promoter variants of the cannabinoid receptor 1 (CNR1) gene and the serotonin transporter gene lead to extremely low or high synaptic serotonin concentrations and, in turn, to an anxious phenotype (Lazary et al, 2009). Another recent study reported a reduction of otherwise treatment-resistant nightmares in PTSD patients upon treatment with a synthetic endocannabinoid receptor agonist (Fraser, 2009), which is supported by an analysis of single-nucleotide polymorphisms in the CNR1 gene suggesting a possible association with PTSD (Lu et al, 2008).
The prominent role of CB1 in fear and anxiety is reflected by its expression pattern in the mammalian brain: CB1 is highly abundant in brain regions involved in fear-related processing including the amygdala, the hippocampus, and the neocortex among others (Marsicano and Lutz, 1999; Marsicano and Kuner, 2008). Among these brain regions, the amygdala has a key role in fear memory and, thus, in various anxiety disorders (for review, see LeDoux, 2000; Pape and Pare, 2010). Among the different nuclei of the amygdala, the lateral (LA), basolateral (BLA), and central nucleus (CeA) represent the key substrates of fear memory (LeDoux, 2000; Pape and Pare, 2010). In the last decades, the CeA was mainly considered as ‘output’ station of the amygdala, relaying information from the LA/BLA to different brain areas responsible for distinct aspects of fear behavior (LeDoux et al, 1988). However, recent studies indicate that the CeA comprises inhibitory circuits enabling the processing of information received by its inputs from LA and BLA as well as from thalamic and cortical areas, which project to the CeA either directly or via intercalated cells (for review, see Ehrlich et al, 2009). Thus, neural circuits within the CeA eventually determine the strength and nature of the fear response during different stages of fear learning and unlearning.
Although it seems conceivable that a neuromodulatory system like the endocannabinoid system, which controls the release of other neurotransmitters including GABA and glutamate, would be ideally positioned in a brain structure with gating functions such as the CeA to exert its effects on fear and anxiety, the existence of CB1 in the CeA remains to be proven. A detailed analysis of CB1 expression in the amygdala by immunohistochemistry revealed high levels of CB1 in lateral and basal nuclei, whereas it appeared absent in the central and medial nuclei of the amygdala (Katona et al, 2001). In the same study, the authors failed to detect cannabinoid effects in the CeA by electrophysiological means (Katona et al, 2001), whereas another recent electrophysiological study reported that endocannabinoids regulate inhibitory transmission in the CeA (Roberto et al, 2010). In line with this finding, other studies detected a faint immunopositive signal in the CeA (Tsou et al, 1998; McDonald and Mascagni, 2001; Patel et al, 2005a).
Therefore, we thought it timely to investigate the possible role of CB1 in CeA. The strategy was to (1) demonstrate CB1 expression immunocytochemically, (2) investigate the endocannabinoid-related electrophysiological phenomena of depolarization-induced suppression of excitation (DSE) and inhibition (DSI) in vitro, (3) use a behavioral fear conditioning paradigm proven to recruit endocannabinoid signaling combined with local pharmacological blockade of CB1 in amygdala in vivo, and (4) test for specificity of observed effects through use of CB1 null mutant mice.
MATERIALS AND METHODS
Animals
Male CB1 null mutant mice (CB1−/−; n=22) (Marsicano et al, 2002), male wild-type littermates (CB1+/+; n=36), and male C57BL/6NCrl mice (n=67) at an age of 6 weeks were single housed under a 12 : 12 h light–dark cycle. All experiments were carried out in accordance with the European Committees Council Directive (86/609/EEC). Protocols were approved by the Bezirksregierung Münster (AZ50.0835.1.0, G53/2005).
Drugs
The AMPA receptor antagonist 2,3-dioxo-6-nitro-1,2,3,4-tetrahydrobenzo[f]quinoxaline-7-sulfonamide disodium salt (DNQX); the NMDA receptor antagonist DL-2-amino-5-phosphonopentanoic acid (DL-AP5), the GABAA receptor antagonist 6-imino-3-(4-methoxyphenyl)-1(6H)-pyridazinebutanoic acid hydrobromide (Gabazine), the GABAB receptor antagonist (2S)-3-[[(1S)-1-(3,4-dichloropheny)ethyl]amino-2-hydroxypropyl] (phenylmethyl) phosphinic acid (CGP55845), and the CB1 antagonist (N-(piperidin-1-yl)-5-(4-iodophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide (AM251) were purchased from Biotrend (Tocris, Köln, Germany).
Immunocytochemistry
Animals were deeply anesthetized with pentobarbital and transcardially perfused with PBS containing heparin (5 U/ ml), followed by 4% PFA solution. Brains were isolated and post-fixed for 24 h at 4 °C in 4% PFA solution, treated with 30% sucrose/PBS solution for 48 h at 4 °C, and stored at −80 °C until use. Brain sections (30 μm thick) were prepared on a Microm HM560 cryostat, and stored at −20 °C in cryoprotection solution (25% glycerin, 25% ethylenglycol, and 50% PBS) until use. All incubation steps were performed in 48-well plates on a wave shaker (Heidolph) at RT if not stated otherwise. Sections were first rinsed in PBS (10 min) and then preincubated in blocking solution (5% normal donkey serum, 2.5% BSA, and 0.3% Triton X-100 in PBS) for 1 h. After blocking, the sections were treated o/n at 4 °C with a polyclonal antibody against CB1 (rabbit anti-CB1; 1 : 500; AF380 from Frontier Science) diluted in low concentrated blocking solution (1% normal donkey serum, 0.1% BSA, and 0.3% Triton X-100 in PBS). On the next day, the sections were washed in PBS-T (PBS/0.1% Triton X-100) five times for 10 min and then incubated for 2 h with the secondary antibody (Cy3-labeled anti-rabbit-IgG from goat, 1 : 250, Jackson ImmunoResearch) diluted in the low concentrated blocking solution. The incubation was followed by three 10-min washing steps in PBS. Sections were counterstained with Hoechst 33258 (2 μg/ μl). After the counterstaining the sections were washed twice for 10 min in PBS, followed by 2 min in distilled water. Sections were mounted on glass slides to dry for 2–4 h at 37 °C. The remaining salt was washed off by dipping the slides for 2 s into distilled water. Finally, the sections were dried overnight in a dust-free environment at RT, and covered with MOWIOL and coverslips. Sections were viewed on a laser-scanning confocal microscope (Nikon eC1 plus, Düsseldorf, Germany) with a × 40 oil immersion objective (HCX PL APO 40X/1.25–0.75, Leica Microsystems, Wetzlar, Germany). Results presented in this study were collected from CB1+/+ and CB1−/− mice (n=4 for total experiment).
Preparation of Amygdala Slices
Mice were anesthetized with isofluorane (5%) and decapitated, and their brains were rapidly removed and put into oxygenated (95% O2, 5% CO2) ice-cold artificial cerebrospinal fluid (ACSF) containing (in mM): 120 NaCl, 2.5 KCl, 1.25 NaH2PO4, 22 NaHCO3, 2 MgSO4, 2 CaCl2, and 15 glucose; pH was 7.35. Coronal slices (250 μm thick) containing the amygdalar complex were cut using a Leica VT-1200S microtome (Leica Microsystems) at 4 °C. Slices were incubated at 34 °C for 20 min and then allowed to equilibrate for at least 1 h at room temperature before commencement of recording.
Recording from Visualized Neurons
Recordings were obtained from visually identified neurons in the amygdala as described previously (Jungling et al, 2008; Sosulina et al, 2010). In brief, CeA neurons were identified using differential interference contrast infrared videomicroscopy (S/W-camera CF8/1; Kappa, Gleichen, Germany; Stuart et al, 1993). Whole-cell patch-clamp recordings were made from CeA neurons at 30 °C, using a patch-clamp amplifier (EPC-7; List Medical Systems, Darmstadt, Germany). Slices were superfused continuously (2.0 ml/ min) with an oxygenated solution identical to one used for cutting the slices. During recording, the majority of cells were filled with biocytin (1%) and then processed by the conventional immunoperoxidase method. The exact location of the cells was determined by light microscopy. Immediately after breaking through for whole-cell patch-clamp, neurons were characterized by electrotonic and electrogenic parameters as reported before (Sosulina et al, 2010). Access resistance was evaluated during recordings upon a 3 mV, 10 ms pulse applied 600 ms after each evoked response. Correction for liquid junction potential changes (10 mV) was applied. Switching frequency was 30 kHz, gain 1.0–1.2. Records were filtered at 3 kHz (eight-pole Bessel filter), sampled at 10 kHz using pClamp9 software (Axon Instruments, Union City, CA), and analyzed off-line using the programs MATLAB 7.0-R14 (The Math Works, Natick, MA) and Clampfit 10.0 (Molecular Devices Corporation).
Electrophysiological Recordings
Coronal slices containing the amygdala were prepared as described above and placed in a submerged chamber, continuously superfused with ACSF at 30 °C. Whole-cell recordings were obtained with micropipettes filled with (in mM): K-Gluconic acid 100, KCl 50, CsCl 10, HEPES 10, EGTA 0.2, MgCl2 1, Mg-ATP 1, Na-GTP 0.3, pH7.3 adjusted with 1 M KOH. EGTA concentration was kept low because of the Ca2+ dependence of DSE or DSI (Lenz and Alger, 1999). Access resistance was continuously monitored and ranged between 4 and 9.5 MΩ. Neurons were recorded in the medial section of the CeA (CeAM; Supplementary Figure S1 and Figures 2a and 3a), and those displaying resting membrane potential positive to –60 mV or changes in capacitive transient >10% during the course of the experiment were discarded. Extracellular stimuli (100 μs, 50–600 μA) were delivered through a bipolar stainless steel electrode placed in the BLA or lateral part of the CeA (CeAL). Glutamatergic and GABAergic components of synaptic responses were isolated by addition of gabazine (1 μM) and CGP55845 (50 μM), and DNQX (10 μM) and AP-5 (50 μM), respectively.
DSE and DSI
DSE or DSI tests were performed every 5 min, and consisted of 30 microstimuli (50 to 600 μA at 0.25 Hz) applied to either BLA or CeAL before, and 75 stimuli after postsynaptic depolarization from −70 to 0 mV. Stimuli were set at 20–30% of maximal synaptic response amplitude, and duration of depolarization was varied between 0.1 and 10 s. For each neuron, at least three tests of DSE or DSI were made, and different durations were tested in a pseudo-randomized fashion. The degree of suppression was calculated using the mean of 15 evoked responses just before the depolarization (amp baseline) and three evoked responses just after depolarization (amp test) using the formula (modified from Wilson and Nicoll, 2001): % of baseline=100*(amp test/amp baseline). For statistical analysis the amplitudes of every three evoked postsynaptic currents in control and after depolarization were averaged and normalized against mean amplitude in control. These normalized values were then averaged over groups of cells. Duration of DSE or DSI was calculated from the sliding average of the amplitude of every three responses plotted as a function of time. Plots were adjusted with a one-phase association equation. Duration of DSE or DSI was defined as the time between the end of the depolarization and the time when evoked currents amplitude returned to control. Some slices were preincubated with the CB1 antagonist AM251 (2 μM) at least 1 h before testing DSE or DSI.
Surgery and Local Drug Application
Mice were implanted bilaterally with a 26-gauge stainless steel guide cannula ending 1.0 mm above CeA (stereotaxic coordinates: 1.0 mm anterior, 2.8 mm lateral, and 3.0 mm ventral from brain surface) or BLA (stereotaxic coordinates: 1.0 mm, 3.1 mm, and 3.3 mm, respectively) under deep pentobarbital anesthesia (75 mg/kg i.p.), as described previously (Jungling et al, 2008). After 1 week of recovery from surgery, vehicle (6.66% dimethylsulfoxide, 6.66% Tween-80 in saline) or AM251 (1 ng or 1 μg in 0.5 μl vehicle) were infused with a 33-gauge beveled needle injector at 0.1 μl/min (0.5 μl each side) under isoflurane anesthesia. Placement of the infusion cannulae was histologically verified, and only animals with proper injection sites in the CeA or in the BLA were included in the analysis (Supplementary Figure S2, for examples please note the histological controls therein).
Fear Conditioning/Extinction
Mice were placed in a fear training apparatus (TSE, Bad Homburg, Germany), as described previously (Kamprath et al, 2006; Plendl and Wotjak, 2010; Sangha et al, 2009). After 3 min, a 20-s tone of 9 kHz and 80 dB (conditioned stimulus, CS+) was presented, which co-terminated with a 2-s scrambled electric foot shock of 0.7 mA (unconditioned stimulus). On the next day (day 1 (d1)), mice were locally treated with AM251 or vehicle, 30 min later transferred to a novel context, and exposed to CS+ for 200 s. For fear extinction, CS+ exposure was repeated on days 2 and 3 (d2 and d3; Figure 5a). Behavioral responses were assessed by a trained observer unaware of the treatment conditions. Freezing was defined as the absence of all movements except for those related to respiration. During analysis, the freezing response was either summarized over the complete 200 s of tone presentation or it was subdivided into ten distinct 20-s intervals, and freezing response was expressed as a percentage of the respective time (‘freezing time’ per interval/total interval time). In two series of experiments, slices were prepared following fear conditioning, either 24 h after fear training (FC-d1) or 24 h after the second series of CS+ exposure (FC-d3), and DSE and DSI were tested as described above.
Elevated Plus Maze (EPM)
The plus maze consisted of two open (30 × 5 cm) and two all-enclosed arms (30 × 5 × 25 cm) connected by a central platform (5 × 5 cm) elevated 75 cm above the floor. Light intensity on the open arms was 250 lux. At the beginning of the experiment, the mouse was placed in the central area facing a closed arm and was then allowed to freely explore the maze for 5 min. Mice were exposed to the maze for a second trial 24 h later. Local drug application was performed 30 min before the first plus maze exposure only. The behavior on the plus maze was monitored by a video motility system (Video-Mot II; TSE, Bad Homburg, Germany), which automatically quantified the total distance moved. Entries and time spent in the open and closed arms, respectively, were scored by a trained observer unaware of the genotype or treatment condition, with the criterion for an arm entry defined as all four paws in an arm of the maze. Percent entries into open arms were expressed as the number of open arm entries/total arm entries × 100. Exclusion criteria embraced freezing over the full time of the test and stereotypic behavior. None of the animals tested met any of the behavioral exclusion criteria.
Statistics
Data were analyzed using Student's t-test and one- or two-way ANOVA for repeated measurements using STATISTICA 8.0 (StatSoft, Tulsa, OK), GraphPad Prism 5.0 (GraphPad Software, San Diego, CA), or PASW Statistics 18 (SPSS, Chicago, IL). Newman–Keuls test was used as post hoc test. Data are presented as mean±SEM. Statistical significance was accepted if p<0.05.
RESULTS
Immunolocalization of CB1 in the CeA
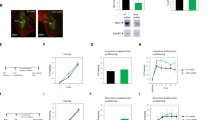
Confocal inspection revealed high levels of CB1 immunofluorescence in BLA, whereas signals were sparse in CeA (Figure 1a), where fiber-like elements bearing beaded protrusions formed CB1 immunoreactive mesh-like patterns mostly in medioventral parts of CeA, with denser networks in the CeAM compared with CeAL (Figure 1a1 and a2). CB1-immunopositive reactions were absent in CB1−/− (Figure 1b and b1).
CB1 immunofluorescence in the amygdala of CB1+/+ (a) and CB1−/− (b) mice. (a) In the amygdala of CB1+/+ mice, we detected high levels of CB1 immunoreactivity in the BLA compared with the LA and CeA, with area displayed at higher magnification in (a1 and a2) as indicated. Note higher density of CB1-positive fibers in medial (a2) compared with lateral (a1) CeA. CB1 immunoreactivity shows up as bead cord-like structures forming a mesh-like pattern. (b) In CB1−/− mice, no CB1 immunofluorescence could be detected. Only a low-level background fluorescence could be observed. (b1) Area indicated in (b) at higher magnification.
Suppression of Synaptic Transmission Mediated via CB1 in CeAM in Vitro
To probe functional CB1 in CeA in vitro, we used depolarization-induced suppression of synaptic transmission, whereby strong postsynaptic depolarization triggers endocannabinoid release and subsequent decrease in transmitter release via activation of presynaptic CB1 (for review, see Chevaleyre et al, 2006). Recordings were obtained from CeAM neurons possessing electrophysiological properties as described previously (Supplementary Figure S1; Dumont et al, 2002). Excitatory (EPSCs) and inhibitory postsynaptic currents (IPSCs) were evoked upon local electrical microstimulation of the BLA and CeAL, respectively (Ehrlich et al, 2009). High failure rates at low stimulation intensities and constant response latencies indicated the monosynaptic nature of the evoked responses. EPSCs were sensitive to ionotropic glutamatergic antagonists (NBQX: 10 μM and DL-AP5: 50 μM; tested in n=6 cells), and IPSCs could be blocked by the GABAA receptor antagonist gabazine (1 μM; n=3). For DSE, neurons were depolarized from −70 mV to 0 mV through direct current injection (duration 10 and 5 s), and the effects on EPSCs evoked upon BLA stimulation were examined (Figure 2a). In all neurons tested (n=9), DSE was significant upon 10 s depolarization, in that EPSCs were reduced from an average amplitude of −62.3±7.6 pA at baseline to 60±3.2% after postsynaptic depolarization (Figure 2b; p=0.001; one-way ANOVA). EPSC amplitudes recovered to baseline within 70.3±6.3 s. In contrast, 5 s depolarization was not sufficient to induce significant DSE in the same cells (90.8±8.8% compared with baseline; n=9), and the maximal change in EPSC amplitude differed significantly following 5 and 10 s depolarization (p=0.0137, unpaired t-test; Figure 2b and d). In age-matched CB1−/−, 10 s depolarization did not induce measurable DSE (97.1±2.5% of baseline; n=10; Figure 2c and d). Moreover, DSE was not detected in CeAM slices from CB1+/+ that had been preincubated with the CB1 antagonist AM251 (2 μM; 100.8±2% of baseline; n=10; Figure 2c and d), and bath application of AM251 blocked the previously established DSE in CeAM neurons (67.3±4.4% before and 101.4±2.6% after AM251; n=5; p<0.05; paired t-test; Figures 2c and d, and 4a and b).
CB1-mediated DSE in CeAM. (a) Recording (R) and stimulation sites (S) in an amygdalar slice. (b) Summarized data of DSE in 9 neurons upon 10 and 5 s depolarization. In this and subsequent panels, the bar indicates the time when the depolarization was applied. Averaged EPSCs before (n=15 traces) and after (n=3 traces) (A) 10 s and (B) 5 s depolarization. Dotted line indicates the latency. (c) Summarized data of DSE (10 s) in control, CB1−/−, and in AM251. (A) Traces taken from AM251 and (B) CB1−/−. (d) Summary of the change in EPSC amplitudes (baseline=100%) for every experimental manipulation. Time of depolarization is depicted. For this and following figure, the number in brackets is the number of neurons recorded. The number in square corresponds to the same neurons. #Compared with baseline; *in between groups; *p<0.05; ###,***p<0.001.
DSI was next investigated using IPSCs evoked upon CeAL stimulation, and testing the effects of postsynaptic depolarization (−70 to 0 mV; Figure 3a). IPSCs displayed a mean amplitude of −136.6±23.2 pA at Vh=−70 mV (n=7), which was significantly reduced upon both 10 and 5 s depolarization in all neurons tested (n=4; 65.1±5.7 and 48.9±6.3% of baseline, respectively; p<0.05; one-way ANOVA; Figure 3b and d), indicating effective DSI. Interestingly, short-duration depolarizations (0.1, 1, and 5 s) tested in a separate group of cells (n=4) were also sufficient to induce DSI (0.1s: 77.6±5.7%, 1 s: 70.5±14.4%, and 5 s: to 70.6±7% of baseline; p<0.01; unpaired t-test; n=4; Figure 3d). Differences in DSI magnitude were only different between shortest and longest depolarizations tested (0.1 vs 10 s; P=0.016, unpaired t-test, Figure 3d). Significant DSI was not detected in CeAM neurons in CB1−/− (97.1±3.3% of baseline, n=4; Figure 3c and d), and application of the CB1 antagonist AM251 (2 μM) blocked established DSI in CB1+/+ in all CeAM neurons tested (n=4; 54.9±5 vs 106.4±7.5% of baseline; p<0.05; one-way ANOVA; Figures 3c and 4c and d). Taken together, these data indicate that CB1 is functional in CeA and capable of suppressing both excitatory and inhibitory synaptic inputs to CeAM neurons.
CB1-mediated DSI in CeAM. (a) Recording (R) and stimulation sites (S) in an amygdalar slice. (b) Same DSI induced by 10 and 5 s depolarization, averaged from recordings in 4 CeAM neurons. Averaged traces of IPSCs are depicted from one neuron before (n=15 traces averaged) and after (n=3 traces) (A) 10 s and (B) 5 s depolarization. Dotted line indicates the latency. (c) Summarized data of DSI (10 s) in control, CB1−/−, and AM251. (d) Summary of the change in IPSC amplitudes (baseline=100%) for every experimental manipulation.
Effects of the CB1 antagonist AM251 on DSE and DSI in CeAM. (a) Time course of a single DSE experiment. Each dot represents one evoked EPSC, tests of DSE (10 s depolarization) are indicated by arrows, examples of EPSCs depicted in (A) and (B). Application of AM251 (2 μM) is indicated by horizontal bar. (A) Averaged traces of EPSCs before (black, n=15) and immediately after (grey; n=3) the (10 s) depolarization in control. (B) Same as in (A) but during AM251 application (black: before, grey: after 5 s depolarization). Note the blockade of DSE and increase in EPSC amplitude with no change in latency during action of AM251. (b) Summary of DSE and the change in EPSC amplitudes (baseline=100%), from five CeAM neurons. (A) Averaged degree of DSE under control conditions (ACSF) and 30 min after application of AM251 (2 μM). (B) Plot of the degree of suppression after 10 s depolarization against the change in EPSC amplitude at 30 min of AM251 application, revealing a linear correlation between these two parameters. Each dot represents data from one CeAM neuron, the square represents the mean from five neurons. (c) Same as in (a) but for DSI. Note the blockade of DSI and increase in IPSC amplitude with no change in latency during action of AM251. (d) Summary of the change in DSI (baseline=100%), from four CeAM neurons (A) and its relationship with the increase in IPSC amplitude and DSI after AM251 application (B). ***p<0.01.
Notably, application of AM251 (2 μM) increased the amplitudes of both EPSCs (by 52±5%, p<0.05; paired t-test; n=5; Figure 4a) and IPSCs (by 42±6.6%, p<0.05; paired t-test; n=4; Figure 4c). When the suppression after 10 s depolarization was plotted against the change produced by AM251 application, a strong correlation between both parameters on either DSE (R=0.8984) or DSI (R=0.8905) (Figure 4b and d, respectively) was found, indicating the presence of an endocannabinoid tone.
Short-Term Effects on Conditioned Fear Behavior via CB1 in CeA
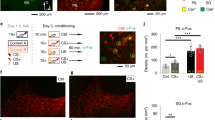
In an auditory fear conditioning paradigm, CB1−/− displayed increased freezing responses in contrast to CB1+/+ (Supplementary Figure S3), with a two-way ANOVA revealing significant genotype and interval effects at all non-reinforced tone presentations, that is, at day 1 (Genotype: P=0.0081; Interval: p<0.0001), day 2 (Genotype: p<0.0001; Interval: p<0.0001), and day 3 (Genotype: p<0.0001; Interval: p<0.0001), which is in line with previous reports indicating impaired fear extinction in CB1−/− (Marsicano et al, 2002; Cannich et al, 2004; Kamprath et al, 2006; Plendl and Wotjak, 2010). To identify the possible contribution of CB1 in CeA, the CB1 antagonist AM251 (1 ng, n=7; 1 μg, n=10) or vehicle (n=10) was injected bilaterally into the CeA in C57BL/6NCrl mice 30 min before the first non-reinforced tone presentation following fear conditioning (Figure 5a and b). A one-way ANOVA of the freezing response revealed a significant drug effect (Drug: P=0.013) between 1 μg AM251 and vehicle group (P=0.0165; Newman–Keuls post hoc test); therefore, this dose of AM251 was used for all following experiments. Detailed analyses showed that injection of AM251 into the CeA resulted in acutely increased fear responses at day 1 (Drug: P=0.0032; Interval: P=0.008, Figure 5c; two-way ANOVA), but not at days 2 or 3 (Figure 5c; statistics not shown). In comparison, local application of AM251 bilaterally into the BLA in the same experimental paradigm, but in a separate batch of animals (n=15; Figure 5d), did not result in significant differences in conditioned fear at day 1, but led to an increased freezing response during the drug-free testing at day 2 (Drug: P=0.0071) and day 3 (Drug: P=0.0028; two-way ANOVA, Figure 5d). This phenotype differs substantially from that obtained upon injections of AM251 into the CeA indicating that CB1 might have different roles in the CeA and BLA in fear memory.
Effects of local application of AM251 in CeA (b, c) and BLA (d) on conditioned freezing. Behavioral protocol is depicted in (a). (b) Effects of local AM251 application in the CeA on CS+-induced freezing at day 1 summarized over the complete 200 s tone presentation. (c) Course of CS+-induced freezing responses at days 1, 2, and 3 (each analyzed in 20-s intervals) upon application of vehicle (open circles) or AM251 (black circles) in CeA. (d) Same as (c) upon application of vehicle (open circles) or AM251 (black circles) in BLA. *p<0.05; **p<0.01.
Short-Term Effects on Anxiety-Related Behavior Mediated via CB1 in CeA
The previous behavioral experiments show a differential involvement of CB1 in CeA vs BLA in fear adaptation processes. Whereas CB1 in BLA appears to be involved in long-term extinction learning processes, CB1 in CeA seems to relate to acute fear adaptation rather than long-term fear learning, and thus might have a role in general anxiety tasks. To test this hypothesis, we exposed a new batch of C57BL/6NCrl mice to the EPM) 30 min after local injection of either 1 μg AM251 (n=13) or vehicle (n=12) bilaterally into the CeA (ie, a similar treatment protocol as used for the fear conditioning experiments). However, no significant effects on anxiety-related parameters (percent time spent on open arms, entries into open arms) could be detected, either at day 1 or on a drug-free second exposure to the EPM at day 2 (Figure 6a and b; statistics not shown). Similarly, the locomotion-related parameters (total distance moved, total entries) did not reveal any significant effects, either at day 1 or at day 2 (Figure 6c and d; statistics not shown).
Effects of local application of AM251 in CeA on elevated plus maze behavior. The effects on the anxiety-related parameters with percent time spent on open arms (a) and percent entries into open arms (b) are shown. The effects on the locomotion-related parameters with total distance moved (c) and total entries (d) are shown.
Altered DSE and DSI in CeA Upon Fear Conditioning
Next, slices were prepared ex vivo from CB1+/+ at day 1 after fear conditioning (FC-d1), and DSE and DSI were assessed in CeAM. Although DSE upon 10 s depolarization did not differ significantly in conditioned (55.4±8.4% of baseline; n=5 neurons from 3 animals) vs naive mice (61.9±2.6%; n=11/5 mice), 5 s depolarization in the same cells resulted in a significant DSE in conditioned animals (61.9±6.4%; p<0.01, unpaired t-test; n=5/3 mice), but failed to evoke DSE in naive controls (90.8±8.8%; n=9; Figure 7a and b). Testing DSI revealed a significant (P=0.022, unpaired t-test) increase in suppression of inhibition upon 0.1 s depolarization in conditioned (60.1±5.6%; n=6/3 mice) compared with naive controls (77.63±5.7%; n=4/3 mice; Figure 7c), whereas DSI upon longer depolarization tested was not different between groups of animals (Figure 7d). Slices prepared from fear-conditioned mice 24 h after the second series of CS+ exposure (FC-d3) revealed no differences in DSE and DSI compared with naive mice. It is noteworthy that depolarization protocols were used in these experiments proven to reveal significant differences in DSE and DSI 24 h after fear conditioning (FC-d1). The magnitude of DSE upon 5 s depolarization in the FC-d3 group (91.2±7.9% of baseline; n=5 neurons from 2 animals) was not different from that in naive mice (P=0.9737), but significantly smaller compared with FC-d1 (p<0.01; unpaired t-test; Figure 7b). A 0.1 s depolarization induced effective DSI (79.8±4.8% of baseline; n=5 neurons from 2 animals; Figure 7b) in the FC-d3 group, similar to that in naive mice (P=0.7701), and significantly different compared with the FC-d1 group (p<0.01, unpaired t-test).
DSE and DSI in CeAM slices prepared from naive animals at 1 and 3 days after fear conditioning (FC-d1 and FC-d3, respectively). (a) DSE upon 5 s depolarization. (b) Summary of the change in EPSC amplitudes upon 5 and 10 s depolarization (baseline=100%). Note enhancement of DSE upon 5 s but not 10 s depolarization in FC-d1 but not in FC-d3. (c) DSI upon 0.1 s depolarization. (d) Summary of the change in IPSC amplitudes at depolarization of different durations (baseline=100%). Note enhancement of DSI at 0.1 s depolarization in FC-d1 but not in FC-d3. #Compared with baseline; *in between groups; *p<0.05; ##p<0.01; ###,***p<0.001.
DISCUSSION
Both DSI and DSE have been described in BLA projection neurons, and have been associated with CB1 activation in axons of GABAergic interneurons (Zhu and Lovinger, 2005; Patel et al, 2009) and putative glutamatergic cortical input fibers (Kodirov et al, 2010), respectively. In the present study, DSI and DSE were observed in CeAM neurons for putatively monosynaptic responses evoked upon stimulation of CeAL and BLA, respectively. Mediation through CB1 was shown by sensitivity toward a CB1 antagonist and absence in CB1−/−. In view of glutamatergic neurons projecting from BLA to CeAM, and a strong GABAergic projection from CeAL to CeAM (Ehrlich et al, 2009), it seems reasonable to suggest that CB1 are located on the respective axonal terminals. Involvement of fibers traversing the BLA and CeAL cannot be excluded, although this possibility would be difficult to reconcile with the local nature of CB1-containing interneuronal axons, the lack of CB1 in intercalated GABAergic neurons (Geracitano et al, 2007), and the observed regional specificity of evoked EPSCs and IPSCs in CeAM. In keeping with this, endocannabinoids spread over only micrometer distances (Wilson and Nicoll, 2001), indicative of a local effect in CeAM. Although the strongest suppression was found upon a 10 s depolarization for both DSI and DSE, shorter depolarization times abolished DSE, but not DSI. This might relate to differences in CB1 expression, as GABAergic neurons express CB1 at higher levels compared with glutamatergic neurons (Marsicano and Lutz, 1999; Ohno-Shosaku et al, 2002), and to differences in endocannabinoid production/availability (Straiker and Mackie, 2005; Straiker and Mackie, 2009). Moreover, blockade of CB1 by AM251 resulted in an increased amplitude of evoked EPSCs and IPSCs, indicating that tonic endocannabinoid signaling (Roberto et al, 2010) or constitutive activity of CB1 (Losonczy et al, 2004) dampens synaptic transmission in CeAM. A similar increase in evoked IPSCs in CeAM upon application of CB1 antagonists has recently been reported, and been related to retrograde endocannabinoid signaling based upon paired-pulse analysis and the blocking effect of postsynaptic Ca2+chelators (Roberto et al, 2010).
Adaptation to a stressor resulted in increased late DSI in the BLA (Patel et al, 2009), letting us hypothesize that the experience of fear conditioning induces changes in endocannabinoid signaling in CeA, which might be reflected in DSE or DSI. A single tone-shock pairing indeed resulted in more efficient DSE and DSI 24 h later, in that shorter postsynaptic depolarization was sufficient to induce near-maximal suppression of synaptic transmission when compared with naive animals. It is noteworthy that the enhanced DSE and DSI had normalized 3 days after fear conditioning, supporting the notion of a short-term adaptation mediated via endocannabinoid signaling in CeAM. Possible underlying mechanisms include increases in endocannabinoid availability, for instance, through regulated de novo synthesis or degradation (for review, see Hillard and Jarrahian, 2003), or increases in CB1 sensitivity or activity. In fact, endocannabinoid levels notably vary in different behavioral tasks, depending on task specificity, stage of adaptation, brain region, and prevailing endocannabinoid (Marsicano et al, 2002; Kamprath et al, 2006; Patel et al, 2005b; Hill et al, 2005; Rademacher et al, 2008).
Mice were subjected to an auditory fear conditioning/extinction protocol previously proven to recruit endocannabinoid signaling during the non-reinforced exposures to the conditioned tone, but not during acquisition of conditioned fear (Marsicano et al, 2002; Kamprath et al, 2006; Plendl and Wotjak, 2010; Niyuhire et al, 2007; see Supplementary Figure S3). Using this protocol, but local infusion of the CB1 antagonist into the CeA before the first tone exposure, yielded a similar increase in subsequent conditioned freezing as previously described with systemic CB1 antagonist application (Marsicano et al, 2002; Kamprath et al, 2006; Plendl and Wotjak, 2010), which was not observed upon drug-free testing on later days. Despite histological verification of infusion sites in the CeA, the pharmacologically active substance might have reached the BLA, which expresses CB1 at high levels. Voting against this possibility is the finding that local application of AM251 bilaterally into the BLA had no acute effect on freezing to conditioned tone at day 1, but resulted in increased freezing upon drug-free testing on later days. These findings indicate a specific role of regionally distributed CB1 in the amygdala in fear memory, with CB1 in BLA being involved in extinction processes in the long-term range, whereas CB1 in CeA contributes to acute fear expression and/or within-session extinction. In this behavioral paradigm, first tone exposure has been previously found to decrease phosphorylation of ERK p44 and calcineurin levels in the BLA of CB1−/− when compared with wild types, whereas levels were increased in the CeA (Cannich et al, 2004), which may relate to the regionally specific role of endocannabinoid signaling for short- and long-term regulation of conditioned fear. Tests of anxiety-related behavior in the EPM test revealed anxiogenic-like effects of CB1 agonist application in CeA (Zarrindast et al, 2008), whereas CB1 antagonists tested over a wide concentration range had no effect (Zarrindast et al, 2008 and this study), making it unlikely that endocannabinoids are endogenously recruited in this test of anxiety. These conclusions are in line with previous findings indicating that repeated homotypic stress produces temporally and anatomically distinct changes in endocannabinoid signaling components within stress-responsive brain regions (Patel and Hillard, 2008). These signals appear to be essential for the process of stress adaptation (Hill et al, 2010), particularly for habituation to the aversive content of the sensory stimulus (Kamprath et al, 2006). It is noteworthy that stress adaptation has been found to differentially involve the two endocannabinoid ligands, N-arachidonylethanolamine (anandamide; AEA) and 2-arachidonoylglycerol (2-AG), such that the AEA content is persistently decreased throughout the corticolimbic stress circuit, whereas 2-AG is exclusively elevated within the amygdala (Hill et al, 2010).
In conclusion, the localization of CB1 in the CeA puts the endocannabinoid system in a near-ideal position to control both inhibitory and excitatory synaptic transmission to the major station of fear-related outputs in the amygdala. This intra-amygdaloid feedback system may complement extra-amygdaloid influences, such as prefrontal cortical inputs regulating inhibitory synaptic signals to the CeA during fear extinction (Amano et al, 2010). As recruitment of endocannabinoids appears to require highly aversive conditions (eg, a strong shock intensity; see Kamprath et al, 2009; also Haller et al, 2004; Naidu et al, 2007), it may take an outstanding position to ensure proper adaptation after traumatic events.
References
Amano T, Unal CT, Pare D (2010). Synaptic correlates of fear extinction in the amygdala. Nat Neurosci 13: 489–494.
Cannich A, Wotjak CT, Kamprath K, Hermann H, Lutz B, Marsicano G (2004). CB1 cannabinoid receptors modulate kinase and phosphatase activity during extinction of conditioned fear in mice. Learn Mem 11: 625–632.
Chevaleyre V, Takahashi KA, Castillo PE (2006). Endocannabinoid-mediated synaptic plasticity in the CNS. Annu Rev Neurosci 29: 37–76.
Chhatwal JP, Davis M, Maguschak KA, Ressler KJ (2005). Enhancing cannabinoid neurotransmission augments the extinction of conditioned fear. Neuropsychopharmacology 30: 516–524.
Chhatwal JP, Ressler KJ (2007). Modulation of fear and anxiety by the endogenous cannabinoid system. CNS Spectr 12: 211–220.
Di Marzo V (2009). The endocannabinoid system: its general strategy of action, tools for its pharmacological manipulation and potential therapeutic exploitation. Pharmacol Res 60: 77–84.
Dumont EC, Martina M, Samson RD, Drolet G, Pare D (2002). Physiological properties of central amygdala neurons: species differences. Eur J Neurosci 15: 545–552.
Ehrlich I, Humeau Y, Grenier F, Ciocchi S, Herry C, Luthi A (2009). Amygdala inhibitory circuits and the control of fear memory. Neuron 62: 757–771.
Fraser GA (2009). The use of a synthetic cannabinoid in the management of treatment-resistant nightmares in posttraumatic stress disorder (PTSD). CNS Neurosci Ther 15: 84–88.
Freund TF, Katona I, Piomelli D (2003). Role of endogenous cannabinoids in synaptic signaling. Physiol Rev 83: 1017–1066.
Geracitano R, Kaufmann WA, Szabo G, Ferraguti F, Capogna M (2007). Synaptic heterogeneity between mouse paracapsular intercalated neurons of the amygdala. J Physiol 585: 117–134.
Haller J, Varga B, Ledent C, Barna I, Freund TF (2004). Context-dependent effects of CB1 cannabinoid gene disruption on anxiety-like and social behaviour in mice. Eur J Neurosci 19: 1906–1912.
Hill MN, McLaughlin RJ, Bingham B, Shrestha L, Lee TT, Gray JM et al (2010). Endogenous cannabinoid signaling is essential for stress adaptation. Proc Natl Acad Sci USA 107: 9406–9411.
Hill MN, Patel S, Carrier EJ, Rademacher DJ, Ormerod BK, Hillard CJ et al (2005). Downregulation of endocannabinoid signaling in the hippocampus following chronic unpredictable stress. Neuropsychopharmacology 30: 508–515.
Hillard CJ, Jarrahian A (2003). Cellular accumulation of anandamide: consensus and controversy. Br J Pharmacol 140: 802–808.
Jungling K, Seidenbecher T, Sosulina L, Lesting J, Sangha S, Clark SD et al (2008). Neuropeptide S-mediated control of fear expression and extinction: role of intercalated GABAergic neurons in the amygdala. Neuron 59: 298–310.
Kamprath K, Marsicano G, Tang J, Monory K, Bisogno T, Di Marzo V et al (2006). Cannabinoid CB1 receptor mediates fear extinction via habituation-like processes. J Neurosci 26: 6677–6686.
Kamprath K, Plendl W, Marsicano G, Deussing JM, Wurst W, Lutz B et al (2009). Endocannabinoids mediate acute fear adaptation via glutamatergic neurons independently of corticotropin-releasing hormone signaling. Genes Brain Behav 8: 203–211.
Katona I, Rancz EA, Acsady L, Ledent C, Mackie K, Hajos N et al (2001). Distribution of CB1 cannabinoid receptors in the amygdala and their role in the control of GABAergic transmission. J Neurosci 21: 9506–9518.
Kodirov SA, Jasiewicz J, Amirmahani P, Psyrakis D, Bonni K, Wehrmeister M et al (2010). Endogenous cannabinoids trigger the depolarization-induced suppression of excitation in the lateral amygdala. Learn Mem 17: 43–49.
Lazary J, Lazary A, Gonda X, Benko A, Molnar E, Hunyady L et al (2009). Promoter variants of the cannabinoid receptor 1 gene (CNR1) in interaction with 5-HTTLPR affect the anxious phenotype. Am J Med Genet B Neuropsychiatr Genet 150B: 1118–1127.
LeDoux JE (2000). Emotion circuits in the brain. Annu Rev Neurosci 23: 155–184.
LeDoux JE, Iwata J, Cicchetti P, Reis DJ (1988). Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. J Neurosci 8: 2517–2529.
Lenz RA, Alger BE (1999). Calcium dependence of depolarization-induced suppression of inhibition in rat hippocampal CA1 pyramidal neurons. J Physiol 521 (Part 1): 147–157.
Losonczy A, Biro AA, Nusser Z (2004). Persistently active cannabinoid receptors mute a subpopulation of hippocampal interneurons. Proc Natl Acad Sci USA 101: 1362–1367.
Lu AT, Ogdie MN, Jarvelin MR, Moilanen IK, Loo SK, McCracken JT et al (2008). Association of the cannabinoid receptor gene (CNR1) with ADHD and post-traumatic stress disorder. Am J Med Genet B Neuropsychiatr Genet 147B: 1488–1494.
Marsicano G, Kuner R (2008). Anatomical distribution of receptors, ligands and enzymes in the brain and in the spinal cord. Circuitries and neurochemistry. In: Köfalvi A (ed) Cannabinoids and the Brain. Springer: New York. pp 161–201.
Marsicano G, Lutz B (1999). Expression of the cannabinoid receptor CB1 in distinct neuronal subpopulations in the adult mouse forebrain. Eur J Neurosci 11: 4213–4225.
Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG et al (2002). The endogenous cannabinoid system controls extinction of aversive memories. Nature 418: 530–534.
McDonald AJ, Mascagni F (2001). Localization of the CB1 type cannabinoid receptor in the rat basolateral amygdala: high concentrations in a subpopulation of cholecystokinin-containing interneurons. Neuroscience 107: 641–652.
Naidu PS, Varvel SA, Ahn K, Cravatt BF, Martin BR, Lichtman AH (2007). Evaluation of fatty acid amide hydrolase inhibition in murine models of emotionality. Psychopharmacology (Berl) 192: 61–70.
Navarro M, Hernandez E, Munoz RM, del Arco I, Villanua MA, Carrera MR et al (1997). Acute administration of the CB1 cannabinoid receptor antagonist SR 141716A induces anxiety-like responses in the rat. NeuroReport 8: 491–496.
Niyuhire F, Varvel SA, Thorpe AJ, Stokes RJ, Wiley JL, Lichtman AH (2007). The disruptive effects of the CB1 receptor antagonist rimonabant on extinction learning in mice are task-specific. Psychopharmacology (Berl) 191: 223–231.
Ohno-Shosaku T, Tsubokawa H, Mizushima I, Yoneda N, Zimmer A, Kano M (2002). Presynaptic cannabinoid sensitivity is a major determinant of depolarization-induced retrograde suppression at hippocampal synapses. J Neurosci 22: 3864–3872.
Pape HC, Pare D (2010). Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear. Physiol Rev 90: 419–463.
Patel S, Cravatt BF, Hillard CJ (2005a). Synergistic interactions between cannabinoids and environmental stress in the activation of the central amygdala. Neuropsychopharmacology 30: 497–507.
Patel S, Hillard CJ (2006). Pharmacological evaluation of cannabinoid receptor ligands in a mouse model of anxiety: further evidence for an anxiolytic role for endogenous cannabinoid signaling. J Pharmacol Exp Ther 318: 304–311.
Patel S, Hillard CJ. (2008). Adaptations in endocannabinoid signaling in response to repeated homotypic stress: a novel mechanism for stress habituation. Eur J Neurosci 27: 2821–2829.
Patel S, Kingsley PJ, Mackie K, Marnett LJ, Winder DG (2009). Repeated homotypic stress elevates 2-arachidonoylglycerol levels and enhances short-term endocannabinoid signaling at inhibitory synapses in basolateral amygdala. Neuropsychopharmacology 34: 2699–2709.
Patel S, Roelke CT, Rademacher DJ, Hillard CJ (2005b). Inhibition of restraint stress-induced neural and behavioural activation by endogenous cannabinoid signalling. Eur J Neurosci 21: 1057–1069.
Plendl W, Wotjak CT (2010). Dissociation of within- and between-session extinction of conditioned fear. J Neurosci 30: 4990–4998.
Rademacher DJ, Meier SE, Shi L, Ho WS, Jarrahian A, Hillard CJ (2008). Effects of acute and repeated restraint stress on endocannabinoid content in the amygdala, ventral striatum, and medial prefrontal cortex in mice. Neuropharmacology 54: 108–116.
Roberto M, Cruz M, Bajo M, Siggins GR, Parsons LH, Schweitzer P (2010). The endocannabinoid system tonically regulates inhibitory transmission and depresses the effect of ethanol in central amygdala. Neuropsychopharmacology 35: 1962–1972.
Sangha S, Narayanan RT, Bergado-Acosta JR, Stork O, Seidenbecher T, Pape HC (2009). Deficiency of the 65 kDa isoform of glutamic acid decarboxylase impairs extinction of cued but not contextual fear memory. J Neurosci 29: 15713–15720.
Sosulina L, Graebenitz S, Pape HC (2010). GABAergic interneurons in the mouse lateral amygdala: a classification study. J Neurophysiol 104: 617–626.
Straiker A, Mackie K (2005). Depolarization-induced suppression of excitation in murine autaptic hippocampal neurones. J Physiol 569: 501–517.
Straiker A, Mackie K (2009). Cannabinoid signaling in inhibitory autaptic hippocampal neurons. Neuroscience 163: 190–201.
Stuart GJ, Dodt HU, Sakmann B (1993). Patch-clamp recordings from the soma and dendrites of neurons in brain slices using infrared video microscopy. Pflugers Arch 423: 511–518.
Suzuki A, Josselyn SA, Frankland PW, Masushige S, Silva AJ, Kida S (2004). Memory reconsolidation and extinction have distinct temporal and biochemical signatures. J Neurosci 24: 4787–4795.
Tsou K, Brown S, Sanudo-Pena MC, Mackie K, Walker JM (1998). Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience 83: 393–411.
Viveros MP, Marco EM, File SE (2005). Endocannabinoid system and stress and anxiety responses. Pharmacol Biochem Behav 81: 331–342.
Wilson RI, Nicoll RA (2001). Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature 410: 588–592.
Wilson RI, Nicoll RA (2002). Endocannabinoid signaling in the brain. Science 296: 678–682.
Wotjak CT (2005). Role of endogenous cannabinoids in cognition and emotionality. Mini Rev Med Chem 5: 659–670.
Zarrindast MR, Sarahroodi S, Arzi A, Khodayar MJ, Taheri-Shalmani S, Rezayof A (2008). Cannabinoid CB1 receptors of the rat central amygdala mediate anxiety-like behavior: interaction with the opioid system. Behav Pharmacol 19: 716–723.
Zhu PJ, Lovinger DM (2005). Retrograde endocannabinoid signaling in a postsynaptic neuron/synaptic bouton preparation from basolateral amygdala. J Neurosci 25: 6199–6207.
Acknowledgements
We thank Elisabeth Boening, Angelika Klinge, Kathrin Foraita, and Elke Nass for excellent technical assistance and Kay Jüngling for critical reading of this paper. This work was supported by a grant from the Deutsche Forschungsgemeinschaft (SFB-TRR58, TP A04 to BL and HCP), and a Max Planck Research Award (to HCP).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Rights and permissions
About this article
Cite this article
Kamprath, K., Romo-Parra, H., Häring, M. et al. Short-Term Adaptation of Conditioned Fear Responses Through Endocannabinoid Signaling in the Central Amygdala. Neuropsychopharmacol 36, 652–663 (2011). https://doi.org/10.1038/npp.2010.196
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2010.196
Keywords
This article is cited by
-
Cannabinoid Type 1 Receptors in the Basolateral Amygdala Regulate ACPA-Induced Place Preference and Anxiolytic-Like Behaviors
Neurochemical Research (2022)
-
The role of endocannabinoids in consolidation, retrieval, reconsolidation, and extinction of fear memory
Pharmacological Reports (2021)
-
Cannabinoid modulation of corticolimbic activation to threat in trauma-exposed adults: a preliminary study
Psychopharmacology (2020)
-
Synapse-specific astrocyte gating of amygdala-related behavior
Nature Neuroscience (2017)
-
Cannabinoid CB1 receptors in distinct circuits of the extended amygdala determine fear responsiveness to unpredictable threat
Molecular Psychiatry (2017)