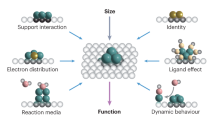
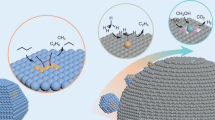
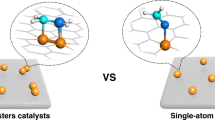
Abstract
Clusters that contain only a small number of atoms can exhibit unique and often unexpected properties. The clusters are of particular interest in catalysis because they can act as individual active sites, and minor changes in size and composition — such as the addition or removal of a single atom — can have a substantial influence on the activity and selectivity of a reaction. Here, we review recent progress in the synthesis and characterization of well-defined subnanometre clusters, and the understanding and exploitation of their catalytic properties. We examine work on size-selected supported clusters in ultrahigh-vacuum environments and under realistic reaction conditions, and explore the use of computational methods to provide a mechanistic understanding of their catalytic properties. We also highlight the potential of size-selected clusters to provide insights into important catalytic processes and their use in the development of novel catalytic systems.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Kappes, M. M., Kunz, R. W. & Schumacher, E. Production of large sodium clusters (Nax, x < 65) by seeded beam expansions. Chem. Phys. Lett. 91, 413–418 (1982).
Ekardt, W. Dynamical polarizability of small metal particles - self-consistent spherical jellium background model. Phys. Rev. Lett. 52, 1925–1928 (1984).
Knight, W. D. et al. Electronic shell structure and abundances of sodium clusters. Phys. Rev. Lett. 52, 2141–2143 (1984).
Kappes, M. M., Radi, P., Schar, M. & Schumacher, E. Probes for electronic and geometrical shell structure effects in alkali-metal clusters. Photoionization measurements on KxLi, KxMg and KxZn (x < 25). Chem. Phys. Lett. 119, 11–16 (1985).
Watanabe, H. I. & Inoshita, T. Superatom: A novel concept in materials science. Optoelectron. Dev. 1, 33–39 (1986).
Jena, P., Khanna, S. N. & Rao, B. K. in Theory of Atomic and Molecular Clusters 27–53 (Springer, 1999).
Bergeron, D. E., Castleman, A. W., Morisato, T. & Khanna, S. N. Formation of Al13I−: Evidence for the superhalogen character of Al13−. Science 304, 84–87 (2004).
Bohme, D. K. & Schwarz, H. Gas-phase catalysis by atomic and cluster metal ions: The ultimate single-site catalysts. Angew. Chem. Int. Ed. 44, 2336–2354 (2005).
Ervin, K., Loh, S. K., Aristov, N. & Armentrout, P. B. Metal cluster ions—The bond-energy of Mn2+. J. Phys. Chem. 87, 3593–3596 (1983).
Smolanoff, J., Lapicki, A. & Anderson, S. L. Use of a quadrupole mass filter for high-energy resolution ion-beam production. Rev. Sci. Instrum. 66, 3706–3708 (1995).
Dietz, T. G., Duncan, M. A., Powers, D. E. & Smalley, R. E. Laser production of supersonic metal cluster beams. J. Chem. Phys. 74, 6511–6512 (1981).
Fayet, P. & Woste, L. Production and study of metal cluster ions. Surf. Sci. 156, 134–139 (1985).
Gantefor, G., Siekmann, H. R., Lutz, H. O. & Meiwes-Broer, K. H. Pure metal and metal-doped rare-gas clusters grown in a pulsed-arc cluster ion-source. Chem. Phys. Lett. 165, 293–296 (1990).
Haberland, H. et al. Filling of micron-sized contact holes with copper by energetic cluster-impact. J. Vac. Sci. Technol. A 12, 2925–2930 (1994).
Lang, S. M., Bernhardt, T. M., Barnett, R. N., Yoon, B. & Landman, U. Hydrogen-promoted oxygen activation by free gold cluster cations. J. Am. Chem. Soc. 131, 8939–8951 (2009).
Schwarz, H. Chemistry with methane: concepts rather than recipes. Angew. Chem. Int. Ed. 50, 10096–10115 (2011).
Dong, F. et al. Experimental and theoretical study of the reactions between neutral vanadium oxide clusters and ethane, ethylene, and acetylene. J. Am. Chem. Soc. 130, 1932–1943 (2008).
Schroder, D. & Schwarz, H. C–H and C–C bond activation by bare transition-metal oxide cations in the gas-phase. Angew. Chem. Int. Ed. 34, 1973–1995 (1995).
Feyel, S., Schroder, D., Rozanska, X., Sauer, J. & Schwarz, H. Gas-phase oxidation of propane and 1-butene with V3O7+: Experiment and theory in concert. Angew. Chem. Int. Ed. 45, 4677–4681 (2006).
Castleman, A. W. Cluster structure and reactions: Gaining insights into catalytic processes. Catal. Lett. 141, 1243–1253 (2011).
Kaden, W. E., Wu, T., Kunkel, W. A. & Anderson, S. L., Electronic structure controls reactivity of size-selected Pd clusters adsorbed on TiO2 surfaces. Science 326, 826–829 (2009).
Lim, D. C., Hwang, C. C., Gantefor, G. & Kim, Y. D. Model catalysts of supported Au nanoparticles and mass-selected clusters. Phys. Chem. Chem. Phys. 12, 15172–15180 (2010).
Palmer, R. E., Pratontep, S. & Boyen, H. G. Nanostructured surfaces from size-selected clusters. Nature Mater. 2, 443–448 (2003).
Bromann, K. et al. Controlled deposition of size-selected silver nanoclusters. Science 274, 956–958 (1996).
Duffe, S. et al. Softlanding and STM imaging of Ag561 clusters on a C60 monolayer. Eur. Phys. J. D 45, 401–408 (2007).
Pearmain, D. et al. Size and shape of industrial Pd catalyst particles using size-selected clusters as mass standards. Appl. Phys. Lett. 102, 163103 (2013).
Popok, V. N., Barke, I., Campbell, E. E. B. & Meiwes-Broer, K.-H. Cluster-surface interaction: From soft landing to implantation. Surf. Sci. Rep. 66, 347–377 (2011).
Lee, S. et al. Selective propene epoxidation on immobilized Au6–10 clusters: The effect of hydrogen and water on activity and selectivity. Angew. Chem. Int. Ed. 121, 1495–1499 (2009).
Lei, Y. et al. Increased silver activity for direct propylene epoxidation via subnanometer size effects. Science 328, 224–228 (2010).
Peters, S. et al. Inner-shell photoionization spectroscopy on deposited metal clusters using soft x-ray synchrotron radiation: An experimental setup. Rev. Sci. Instrum. 80, 125106 (2009).
Abbet, S., Sanchez, A., Heiz, U. & Schneider, W. D. Tuning the selectivity of acetylene polymerization atom by atom. J. Catal. 198, 122–127 (2001).
Lee, S. et al. Support-dependent performance of size-selected subnanometer cobalt cluster-based catalysts in the dehydrogenation of cyclohexene. ChemCatChem 4, 1632–1637 (2012).
Heiz, U., Vanolli, F., Sanchez, A. & Schneider, W. D. Size-dependent molecular dissociation on mass-selected, supported metal clusters. J. Am. Chem. Soc. 120, 9668–9671 (1998).
Cox, D. M. et al. Electronic structure of deposited monosized metal-clusters. Z. Phys. D Atom. Mol. Clusters. 20, 385–386 (1991).
Anderson, S. L., Mizushima, T. & Udagawa, Y. Growth restructuring of Pd clusters induced by CO adsorption. J. Phys. Chem. 95, 6603–6610 (1991).
Deng, W. et al. Cleavage of the C-O-C bond on size-selected subnanometer cobalt catalysts and on ALD-cobalt coated nanoporous membranes. Appl. Catal. A 393, 29–35 (2011).
Vajda, S. et al. Subnanometre platinum clusters as highly active and selective catalysts for the oxidative dehydrogenation of propane. Nature Mater. 8, 213–216 (2009).
Kwon, G. et al. Size-dependent subnanometer Pd cluster (Pd4, Pd6, and Pd17) water oxidation electrocatalysis. ACS Nano 7, 5808–5817 (2013).
Lu, J. C. et al. Effect of the size-selective silver clusters on Li2O2 morphology in lithium-oxygen batteries. Nature Commun. 5, 4895 (2014).
Haruta, M., Kobayashi, T., Sano, H. & Yamada, N. Novel gold catalysts for the oxidation of carbon-monoxide at a temperature far below 0° C. Chem. Lett. 16, 405–408 (1987).
Choudhary, T. V., Sivadinarayana, C., Datye, A. K., Kumar, D. & Goodman, D. W. Acetylene hydrogenation on Au-based catalysts. Catal. Lett. 86, 1–8 (2003).
Haruta, A. When gold is not noble: Catalysis by nanoparticles. Chem. Rec. 3, 75–87 (2003).
Fu, Q., Saltsburg, H. & Flytzani-Stephanopoulos, M. Active nonmetallic Au and Pt species on ceria-based water-gas shift catalysts. Science 301, 935–938 (2003).
Ferrando, R., Jellinek, J. & Johnston, R. L. Nanoalloys: From theory to applications of alloy clusters and nanoparticles. Chem. Rev. 108, 845–910 (2008).
Thomas, J. M., Johnson, B. F. G., Raja, R., Sankar, G. & Midgley, P. A. High-performance nanocatalysts for single-step hydrogenations. Acc. Chem. Res. 36, 20–30 (2003).
Raja, R. et al. Preparation and characterisation of a highly active bimetallic (Pd-Ru) nanoparticle heterogeneous catalyst. Chem. Commun. 1999, 1571–1572 (1999).
Kimura, K. et al. Size determination of gold clusters by polyacrylamide gel electrophoresis in a large cluster region. J. Phys. Chem. C 113, 14076–14082 (2009).
Knoppe, S., Boudon, J., Dolamic, I., Dass, A. & Burgi, T. Size exclusion chromatography for semipreparative scale separation of Au38(SR)24 and Au40(SR)24 and larger clusters. Anal. Chem. 83, 5056–5061 (2011).
Li, G. & Jin, R. C. Atomically precise gold nanoclusters as new model catalysts. Acc. Chem. Res. 46, 1749–1758 (2013).
Somorjai, G. A., Contreras, A. M., Montano, M. & Rioux, R. M. Clusters, surfaces, and catalysis. Proc. Natl Acad. Sci. USA 103, 10577–10583 (2006).
Muetterties, E. L. Molecular metal clusters. Science 196, 839–848 (1977).
Tao, F. et al. Break-up of stepped platinum catalyst surfaces by high CO coverage. Science 327, 850–853 (2010).
Wegner, K., Piseri, P., Tafreshi, H. V. & Milani, P. Cluster beam deposition: a tool for nanoscale science and technology. Phys. D: Appl. Phys. 39, R439–R459 (2006).
Kroto, H. W., Heath, J. R., O'Brien, S. C., Curl, R. F. & Smalley, R. E. C60: Buckminsterfullerene. Nature 318, 162–163 (1985).
Nonose, S., Sone, Y., Onodera, K., Sudo, S. & Kaya, K. Structure and reactivity of bimetallic ConVm clusters. J. Phys. Chem. 94, 2744–2746 (1990).
Bouwen, W. et al. Production of bimetallic clusters by a dual-target dual-laser vaporization source. Rev. Sci. Instrum. 71, 54–58 (2000).
Schmidt, M., Kusche, R., von Issendorff, B. & Haberland, H. Irregular variations in the melting point of size-selected atomic clusters. Nature 393, 238–240 (1998).
Yasumatsu, H. Generation of intense and cold beam of Pt-Ag bi-element cluster ions having single-composition. Eur. Phys. J. D 63, 195–200 (2011).
Yin, F., Wang, Z. W. & Palmer, R. E. Controlled formation of mass-selected Cu-Au core-shell cluster beams. J. Am. Chem. Soc. 133, 10325–10327 (2011).
Pratontep, S., Carroll, S. J., Xirouchaki, C., Streun, M. & Palmer, R. E. Size-selected cluster beam source based on radio frequency magnetron plasma sputtering and gas condensation. Rev. Sci. Instrum. 76, 045103 (2005).
Bansmann, J. et al. Temperature dependent magnetic spin and orbital moments of mass-filtered cobalt clusters on Au(111). Eur. Phys. J. D 45, 521–528 (2007).
Rosellen, W., Bettermann, H., Veltum, T. & Getzlaff, M. Low energy impact of size selected FeCo nanoparticles with a W(110) surface. Physica E 44, 1683–1686 (2012).
Li, A. et al. Using ambient ion beams to write nanostructured patterns for surface enhanced Raman spectroscopy. Angew. Chem. Int. Ed. 53, 12528–12531 (2014).
Yamashita, M. & Fenn, J. B. Electrospray ion-source — another variation on the free-jet theme. J. Phys. Chem. 88, 4451–4459 (1984).
Johnson, G. E., Priest, T. & Laskin, J. Charge retention by gold clusters on surfaces prepared using soft landing of mass selected ions. ACS Nano 6, 573–582 (2012).
Schroder, D., Weiske, T. & Schwarz, H. Dissociation behavior of Cu(urea)+ complexes generated by electrospray ionization. Int. J. Mass Spectrom. 219, 729–738 (2002).
Dietl, N. et al. Structure and chemistry of the heteronuclear oxo-cluster VPO4•+: A model system for the gas-phase oxidation of small hydrocarbons. J. Am. Chem. Soc. 135, 3711–3721 (2013).
von Issendorff, B. & Palmer, R. E. A new high transmission infinite range mass selector for cluster and nanoparticle beams. Rev. Sci. Instrum. 70, 4497–4501 (1999).
Miller, P. E. & Denton, M. B. The quadrupole mass filter — Basic operating concepts. J. Chem. Edu. 63, 617–622 (1986).
Methling, R. P. et al. Magnetic studies on mass-selected iron particles. Eur. Phys. J. D 16, 173–176 (2001).
de Heer, W. A. The physics of simple metal clusters: experimental aspects and simple models. Rev. Mod. Phys. 65, 611–676 (1993).
Yin, C. T., Tyo, E. C. & Vajda, S. in Heterogeneous Catalysis at the Nanoscale for Energy Applications (eds Tao, F., Schneider, W. F. & Kamat, P.) Ch. 3 (Wiley, 2014).
Hovel, H. et al. Controlled cluster condensation into preformed nanometer-sized pits. J. Appl. Phys. 81, 154–158 (1997).
Dietsche, R. et al. Comparison of electronic structures of mass-selected Ag clusters and thermally grown Ag islands on sputter-damaged graphite surfaces. Appl. Phys. A 90, 395–398 (2008).
Schouteden, K. et al. Probing the atomic structure of metallic nanoclusters with the tip of a scanning tunneling microscope. Nanoscale 6, 2170–2176 (2014).
Bonanni, S., Ait-Mansour, K., Harbich, W. & Brune, H. Effect of the TiO2 reduction state on the catalytic CO oxidation on deposited size-selected Pt clusters. J. Am. Chem. Soc. 134, 3445–3450 (2012).
Carroll, S. J. et al. Pinning of size-selected Ag clusters on graphite surfaces. J. Chem. Phys. 113, 7723–7727 (2000).
Yin, F., Lee, S., Abdela, A., Vajda, S. & Palmer, R. E. Communication: Suppression of sintering of size-selected Pd clusters under realistic reaction conditions for catalysis. J. Chem. Phys. 134, 141101–141104 (2011).
Tong, X. et al. Intact size-selected Aun clusters on a TiO2(110)-(1 × 1) surface at room temperature. J. Am. Chem. Soc. 127, 13516–13518 (2005).
Duffe, S. et al. Penetration of thin C60 films by metal nanoparticles. Nature Nanotech. 5, 335–339 (2010).
Vandamme, N., Janssens, E., Vanhoutte, F., Lievens, P. & Van Haesendonck, C. Scanning probe microscopy investigation of gold clusters deposited on atomically flat substrates. J. Phys. Condens. Matter 15, S2983–S2999 (2003).
Popescu, R. et al. Coarsening of mass-selected Au clusters on amorphous carbon at room temperature. Surf. Sci. 603, 3119–3125 (2009).
Uzun, A., Ortalan, V., Hao, Y. L., Browning, N. D. & Gates, B. C. Nanoclusters of gold on a high-area support: Almost uniform nanoclusters imaged by scanning transmission electron microscopy. ACS Nano 3, 3691–3695 (2009).
Kunz, S. et al. Size-selected clusters as heterogeneous model catalysts under applied reaction conditions. Phys. Chem. Chem. Phys. 12, 10288–10291 (2010).
Mao, B. H. et al. Oxidation and reduction of size-selected subnanometer Pd clusters on Al2O3 surface. J. Chem. Phys. 138, 214304 (2013).
Yoon, B. et al. Charging effects on bonding and catalyzed oxidation of CO on Au8 clusters on MgO. Science 307, 403–407 (2005).
Gilb, S., Arenz, M. & Heiz, U. The polymerization of acetylene on supported metal clusters. Low Temp. Phys. 32, 1097–1103 (2006).
Fan, C., Wu, T., Kaden, W. E. & Anderson, S. L. Cluster size effects on hydrazine decomposition on Irn/Al2O3/NiAl(110). Surf. Sci. 600, 461–467 (2006).
Sanchez, A. et al. When gold is not noble: Nanoscale gold catalysts. J. Phys. Chem. A 103, 9573–9578 (1999).
Lim, D. C., Dietsche, R., Bubek, M., Gantefor, G. & Kim, Y. D. Oxidation and reduction of mass-selected au clusters on SiO2/Si. ChemPhysChem 7, 1909–1911 (2006).
Lim, D. C. et al. Chemistry of mass-selected Au clusters deposited on sputter-damaged HOPG surfaces: The unique properties of Au8 clusters. Chem. Phys. Lett. 439, 364–368 (2007).
Wu, T., Kaden, W. E., Kunkel, W. A. & Anderson, S. L. Size-dependent oxidation of Pdn (n ≤ 13) on alumina/NiAl(110): Correlation with Pd core level binding energies. Surf. Sci. 603, 2764–2770 (2009).
Pacchioni, G. Nanocatalysis: Staying put. Nature Mater. 8, 167–168 (2009).
Baldansuren, A., Dilger, H., Eichel, R.d.-A., van Bokhoven, J. A. & Roduner, E. Interaction and reaction of ethylene and oxygen on six-atom silver clusters supported on LTA zeolite. J. Phys. Chem. C 113, 19623–19632 (2009).
Sungsik, L., Byeongdu, L., Seifert, S., Vajda, S. & Winans, R. E. Simultaneous measurement of X-ray small angle scattering, absorption and reactivity: A continuous flow catalysis reactor. Nucl. Instrum. Methods Phys. Res. Sect. A 649, 200–203 (2011).
Nesselberger, M. et al. The effect of particle proximity on the oxygen reduction rate of size-selected platinum clusters. Nature Mater. 12, 919–924 (2013).
Perez-Alonso, F. J. et al. The effect of size on the oxygen electroreduction activity of mass-selected platinum nanoparticles. Angew. Chem. Int. Ed. 51, 4641–4643 (2012).
Hartl, K. et al. Electrochemically induced nanocluster migration. Electrochim. Acta 56, 810–816 (2010).
Negreiros, F. R. et al. A first-principles theoretical approach to heterogeneous nanocatalysis. Nanoscale 4, 1208–1219 (2012).
Kim, H. Y., Lee, H. M. & Metiu, H. Oxidative dehydrogenation of methanol to formaldehyde by a vanadium oxide cluster supported on rutile TiO2(110): Which oxygen is involved? J. Phys. Chem. C 114, 13736–13738 (2010).
Huber, B., Koskinen, P., Hakkinen, H. & Moseler, M. Oxidation of magnesia-supported Pd-clusters leads to the ultimate limit of epitaxy with a catalytic function. Nature Mater. 5, 44–47 (2006).
Remediakis, I. N., Lopez, N. & Nørskov, J. K. CO oxidation on rutile-supported Au nanoparticles. Angew. Chem. Int. Ed. 117, 1858–1860 (2005).
Molina, L. M. & Hammer, B. Active role of oxide support during CO oxidation at Au/MgO. Phys. Rev Lett. 90, 206102 ( 2003).
Qiao, B. et al. Single-atom catalysis of CO oxidation using Pt1/FeOx . Nature Chem. 3, 634–641 (2011).
Joshi, A. M., Delgass, W. N. & Thomson, K. T. Mechanistic implications of Aun/Ti-lattice proximity for propylene epoxidation. J. Phys. Chem. C 111, 7841–7844 (2007).
Tsunoyama, H., Negishi, Y. & Tsukuda, T. Chromatographic isolation of “missing” Au55 clusters protected by alkanethiolates. J. Am. Chem. Soc. 128, 6036–6037 (2006).
Selva, J. et al. Silver sub-nanoclusters electrocatalyze ethanol oxidation and provide protection against ethanol toxicity in cultured mammalian cells. J. Am. Chem. Soc. 132, 6947–6954 (2010).
Goellner, J. F., Guzman, J. & Gates, B. C. Synthesis and structure of tetrairidium clusters on TiO2 powder: Characterization by infrared and extended X-ray absorption fine structure spectroscopies. J. Phys. Chem. B 106, 1229–1238 (2002).
Corma, A. et al. Exceptional oxidation activity with size-controlled supported gold clusters of low atomicity. Nature Chem. 5, 775–781 (2013).
Acknowledgements
The authors acknowledge the support by the US Department of Energy under Contract DE-AC02-06CH11357 from the Division of Materials Science and Engineering, Basic Energy Sciences, Office of Science.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Tyo, E., Vajda, S. Catalysis by clusters with precise numbers of atoms. Nature Nanotech 10, 577–588 (2015). https://doi.org/10.1038/nnano.2015.140
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nnano.2015.140
This article is cited by
-
Core–shell CoN@Co ultra-stable nanoparticles on biochar for contamination remediation in water and soil
Carbon Research (2024)
-
Core atoms escape from the shell: reverse segregation of Pb–Al core–shell nanoclusters via nanoscale melting
Discover Nano (2023)
-
The highest oxidation state observed in graphene-supported sub-nanometer iron oxide clusters
Communications Chemistry (2023)
-
Advances in heterogeneous single-cluster catalysis
Nature Reviews Chemistry (2023)
-
Scalable synthesis of Cu clusters for remarkable selectivity control of intermediates in consecutive hydrogenation
Nature Communications (2023)