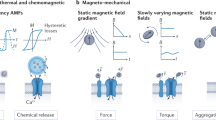
Abstract
By binding magnetic nanoparticles to the surface of cells, it is possible to manipulate and control cell function with an external magnetic field. The technique of activating cells with magnetic nanoparticles offers a means to isolate and explore cellular mechanics and ion channel activation to gain better understanding of these processes. Here, we go beyond using this technique as an investigative tool and focus on its potential to actively control cellular functions and processes with an eye towards biological and clinical applications. In particular, we focus on applications in tissue engineering and regenerative medicine.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Seifritz, W. An elastic value of the protoplasm with further observations on the viscosity of the protoplasm. Brit. J. Exper. Biol. 2, 1–11 (1924).
Heilbrunn, L. V. Eine neue Methode zur Bestimmung der Viskosität lebender Protoplasten. Jahrb. Wiss. Bot. 61, 284 (1922).
Crick, F. H. C. & Hughes, A. F. W. The physical properties of cytoplasm: a study by means of the magnetic particle method. Exp. Cell Res. 1, 37–80 (1950).
Valberg, P. A. & Albertini, D. F. Cytoplasmic motions, rheology and structure probed by a novel magnetic particle method. J. Cell Biol. 101, 130–140 (1985).
Valberg, P. A. & Feldman, H. A. Magnetic particle motions within living cells. Measurement of cytoplasmic viscosity and motile activity. Biophys. J. 52, 551–569 (1987).
Valberg, P. A. & Butler J. P. Magnetic particle motions within living cells. Physical theory and techniques. Biophys. J. 52, 537–550 (1987).
Wang, N., Butler, J. P. & Ingber, D. E. Mechanotransduction across the cell surface and through the cytoskeleton. Science 260, 1124–1127 (1993).
Wang N. & Ingber, D. E. Probing transmembrane mechanical coupling and cytomechanics using magnetic twisting cytometry. Biochem. Cell Biol. 73, 327–35 (1995).
Ingber, D. E. Mechanical control of cyclic cAMP signalling and gene transcription through integrins. Nat. Cell Biol. 2, 666–668 (2000).
Pommerenke, H. et al. Stimulation of integrin receptors using a magnetic drag force device induces intracellular free calcium response. Eur. J. Cell Biol. 70, 157–164 (1996).
Bausch, A. R., Hellerer, U., Essler, M., Aepfelbacher, M. & Sackmann, E. Rapid stiffening of integrin receptor-actin linkages in endothelial cells stimulated with thrombin: A magnetic bead microrheology study. Biophys. J. 80, 2649–2657 (2001).
Bausch, A. R., Moller, W. & Sackmann, E. Measurement of local viscoelasticity and forces in living cells by magnetic tweezers. Biophys. J. 76, 573–579 (1999).
Glogauer, M., Ferrier, J. & McCulloch, C. A. G. Magnetic fields applied to collagen coated beads induce stretch-activated Ca2+ flux in fibroblasts. Am. J. Physiol. 38, C1093–C1104 (1995).
Glogauer, M. & Ferrier, J. A new method for application of force to cells via ferric oxide beads. Eur. J. Physiol. 435, 320–327 (1998).
Pankhurst, Q. A., Connoly, J., Jones, S. K. & Dobson J. Applications of magnetic nanoparticles in biomedicine. J. Phys. D 36, R167–R181 (2003).
Hughes, S., El Haj, A. J. & Dobson, J. Magnetic micro- and nanoparticle mediated activation of mechanosensitive ion channels. Med. Eng. Phys. 25, 754–762 (2005).
Waigh, T. A. Microrheology of complex fluids. Rep. Prog. Phys. 68, 685–742 (2005).
El Haj, A. J., Hughes, S. & Dobson, J. Manipulation of ion channels using magnetic micro- and nanoparticle cytometry. Comp. Biochem. Phys. A 134, S110 (2003).
Hughes, S., McBain, S., Dobson, J. & El Haj, A. J. Selective activation of mechanosensitive ion channels using magnetic particles. J. Roy. Soc. Interface doi:10.1098/rsif.2007.1274(2007).
Howard, J. & Hudspeth, A. J. Compliance of the hair bundle associated with gating of mechanoelectrical transduction channels in the bullfrog's saccular hair cell. Neuron 1, 189–199 (1988).
Walker, L. M. et al. Mechanical manipulation of bone and cartilage cells with optical tweezers. FEBS Lett. 459, 39–42 (1999).
Mannix, R. J., Kumar, S., Cassiola, F., Montoya-Zavala, M. & Ingber, D. E. Nanomagnetic actuation of receptor-mediated signal transduction. Nature Nanotech. 3, 36–40 (2008).
Matthews, B. D., LaVan, D. A., Overby, D. R., Karavitis, J. & Ingberg, D. E. Electromagnetic needles with submicron pole tip radii for nanomanipulation of biomolecules and living cells. Appl. Phys. Lett. 85, 2968–2970 (2004).
Berry, C. C. & Curtis, A. S. G. Functionalisation of magnetic nanoparticles for applications in biomedicine. J. Phys. D 36, R198–R206 (2003).
Neuberger, T., Schopf, B., Hofmann, H., Hofmann, M. & von Rechenberg, B. Superparamagnetic nanoparticles for biomedical applications: Possibilities and limitations of a new drug delivery system. J. Magn. Magn. Mater. 293, 483–496 (2005).
Maheshwari, G., Brown, G., Lauffenburger, D. A., Wells, A. & Griffith, L. G. Cell adhesion and motility depend on nanoscale RGD clustering. J. Cell Sci. 113, 1677–1686 (2000).
Cavalcanti-Adam, E. A. et al. Lateral spacing of integrin ligands influences cell spreading and focal adhesion assembly. Eur. J. Cell Biol. 85, 219–224 (2006).
Cavalcanti-Adam, E. A. et al. Cell spreading and focal adhesion dynamics are regulated by spacing of integrin ligands. Biophys. J. 92, 2964–2974 (2007).
Polte, T. R. et al. Nanostructured magnetizable materials that switch cells between life and death. Biomaterials 28, 2783–2790 (2007).
Guldberg, R. E. et al. Mechanical stimulation of tissue repair in the hydraulic bone chamber J. Bone Miner. Res. 12, 1295–302 (1997).
Walker L. M., Publicover, S. J., Preston, M. R., Said Ahmed, M. A. A. & El Haj, A. J. Calcium channel activation and matrix protein up-regulation in bone cells in response to mechanical strain. J. Cell. Biochem. 79, 648–61 (2000).
Wright, M., Jobanputra, P., Bavington, C., Salter, D. M. & Nuki, G. Effects of intermittent pressure-induced strain on the electrophysiology of cultured human chondrocytes: Evidence for the presence of stretch-activated membrane ion channels. Clinical Sci. 90, 61–71 (1996).
Dobson, J., Keramane, A. & El Haj, A. J. Theory and applications of a magnetic force bioreactor. Eur. Cells Mater. 4 (Suppl. 2), 130–131 (2002).
Cartmell, S. H., Dobson, J., Verschueren, S. & El Haj, A. J. Development of magnetic particle techniques for long-term culture of bone cells with intermittent mechanical activation. IEEE Trans. NanoBiosci. 1, 92–97 (2002).
Dobson, J., Keramane, A. & El Haj, A. J. Theory and applications of a magnetic force bioreactor. Eur. Cells Mater. 4 (Suppl. 2), 130–131 (2002).
Dobson, J., Cartmell, S. H., Keramane, A. & El Haj, A. J. A magnetic force mechanical conditioning bioreactor for tissue engineering, stem cell conditioning and dynamic in vitro screening. IEEE Trans. NanoBiosci. 5, 173–177 (2006).
Hughes, S., Dobson, J. & El Haj, A. J. Magnetic targeting of mechanosensors in bone cells for tissue engineering applications. J. Biomechanics 40, S96–S104 (2007).
Sura, H. S., Magnay J., Dobson J. & El Haj A. J. Gene expression in stem cells following stimulation using magnetic particle technology. Tissue Eng. 13, 1699 (2007).
Kirkham, G. R. et al. Electrophysiological responses of HMSCs and bone cells to magnetic particle tagging. Tissue Eng. 13, 1684 (2007).
Lee, I., Wang, J. H., Lee, Y. T. & Young, T. H. The differentiation of mesenchymal stem cells by mechanical stress or/and co-culture system. Biochem. Biophys. Res. Comm. 352, 147–152 (2007).
Alsberg, E., Feinstein, E., Joy, M. P., Prentiss, M. & Ingber, D. E. Magnetically-guided self-assembly of fibrin matrices with ordered nano-scale structure for tissue engineering. Tissue Eng. 12, 3247–3256 (2006).
Ito, A. et al. Construction and delivery of tissue-engineered human retinal pigment epithelial cell sheets, using magnetite nanoparticles and magnetic force. Tissue Eng. 11, 489–496 (2005).
Ito, A., Ino, K., Kobayashi, T. & Honda, H. The effect of RGD peptide-conjugated magnetite cationic liposomes on cell growth and cell sheet harvesting. Biomaterials 26, 6185–6193 (2005).
Ito, A. et al. Novel methodology for fabrication of tissue-engineered tubular constructs using magnetite nanoparticles and magnetic force. Tissue Eng. 11, 1553–1561 (2005).
Pislaru, S. V. et al. Magnetic forces enable rapid endothelialization of synthetic vascular grafts. Circulation 114, I314–I318 (2006).
Perea, H., Aigner, J., Hopfner, U. & Wintermantel, E. Direct magnetic tubular cell seeding: A novel approach for vascular tissue engineering. Cells Tiss. Org. 183, 156–165 (2006).
Shimizu, K. et al. Effective cell-seeding technique using magnetite nanoparticles and magnetic force onto decellularized blood vessels for vascular tissue engineering. J. Biosci. Bioeng. 103, 472–478 (2007).
Acknowledgements
The author is supported by a Royal Society Wolfson Research Merit Award and wishes to thank J. F. Collingwood and S. F. Dobson for critical readings of early versions of the manuscript.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Dobson, J. Remote control of cellular behaviour with magnetic nanoparticles. Nature Nanotech 3, 139–143 (2008). https://doi.org/10.1038/nnano.2008.39
Issue Date:
DOI: https://doi.org/10.1038/nnano.2008.39
This article is cited by
-
Self-rectifying magnetoelectric metamaterials for remote neural stimulation and motor function restoration
Nature Materials (2024)
-
Recent progress in the effect of magnetic iron oxide nanoparticles on cells and extracellular vesicles
Cell Death Discovery (2023)
-
Remote closed-loop stabilization of robotic Euler–Bernoulli manipulator through predictor feedback
International Journal of Intelligent Robotics and Applications (2023)
-
Modulating cell signalling in vivo with magnetic nanotransducers
Nature Reviews Methods Primers (2022)
-
Detection of magnetic tracers with Mx atomic magnetometer for application to blood velocimetry
Scientific Reports (2021)