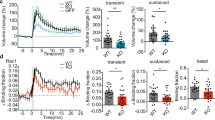
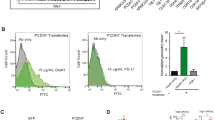
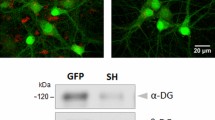
Abstract
The development of dendritic spines is thought to be crucial for synaptic plasticity. Dendritic spines are retracted upon Eph receptor A4 (EphA4) activation, but the mechanisms that control this process are not well understood. Here we report an important function of cyclin-dependent kinase 5 (Cdk5) in EphA4-dependent spine retraction in mice. We found that blocking Cdk5 activity inhibits ephrin-A1–triggered spine retraction and reduction of mEPSC frequency at hippocampal synapses. The activation of EphA4 resulted in the recruitment of Cdk5 to EphA4, leading to the tyrosine phosphorylation and activation of Cdk5. EphA4 and Cdk5 then enhanced the activation of ephexin1, a guanine-nucleotide exchange factor that regulates activation of the small Rho GTPase RhoA. The association between EphA4 and ephexin1 was significantly reduced in Cdk5−/− brains and Cdk5-dependent phosphorylation of ephexin1 was required for the ephrin-A1–mediated regulation of spine density. These findings suggest that ephrin-A1 promotes EphA4-dependent spine retraction through the activation of Cdk5 and ephexin1, which in turn modulates actin cytoskeletal dynamics.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Ethell, I.M. & Pasquale, E.B. Molecular mechanisms of dendritic spine development and remodeling. Prog. Neurobiol. 75, 161–205 (2005).
Henkemeyer, M., Itkis, O.S., Ngo, M., Hickmott, P.W. & Ethell, I.M. Multiple EphB receptor tyrosine kinases shape dendritic spines in the hippocampus. J. Cell Biol. 163, 1313–1326 (2003).
Contractor, A. et al. Trans-synaptic Eph receptor-ephrin signaling in hippocampal mossy fiber LTP. Science 296, 1864–1869 (2002).
Grunwald, I.C. et al. Hippocampal plasticity requires postsynaptic ephrinBs. Nat. Neurosci. 7, 33–40 (2004).
O'Leary, D.D. & Wilkinson, D.G. Eph receptors and ephrins in neural development. Curr. Opin. Neurobiol. 9, 65–73 (1999).
Wilkinson, D.G. Multiple roles of EPH receptors and ephrins in neural development. Nat. Rev. Neurosci. 2, 155–164 (2001).
Gerlai, R. Eph receptors and neural plasticity. Nat. Rev. Neurosci. 2, 205–209 (2001).
Henderson, J.T. et al. The receptor tyrosine kinase EphB2 regulates NMDA-dependent synaptic function. Neuron 32, 1041–1056 (2001).
Murai, K.K., Nguyen, L.N., Irie, F., Yamaguchi, Y. & Pasquale, E.B. Control of hippocampal dendritic spine morphology through ephrin-A3/EphA4 signaling. Nat. Neurosci. 6, 153–160 (2003).
Dalva, M.B. et al. EphB receptors interact with NMDA receptors and regulate excitatory synapse formation. Cell 103, 945–956 (2000).
Penzes, P. et al. Rapid induction of dendritic spine morphogenesis by trans-synaptic ephrinB-EphB receptor activation of the Rho-GEF kalirin. Neuron 37, 263–274 (2003).
Murai, K.K. & Pasquale, E.B. New exchanges in eph-dependent growth cone dynamics. Neuron 46, 161–163 (2005).
Nakayama, A.Y., Harms, M.B. & Luo, L. Small GTPases Rac and Rho in the maintenance of dendritic spines and branches in hippocampal pyramidal neurons. J. Neurosci. 20, 5329–5338 (2000).
Irie, F. & Yamaguchi, Y. EphB receptors regulate dendritic spine development via intersectin, Cdc42 and N-WASP. Nat. Neurosci. 5, 1117–1118 (2002).
Morabito, M.A., Sheng, M. & Tsai, L.H. Cyclin-dependent kinase 5 phosphorylates the N-terminal domain of the postsynaptic density protein PSD-95 in neurons. J. Neurosci. 24, 865–876 (2004).
Zukerberg, L.R. et al. Cables links Cdk5 and c-Abl and facilitates Cdk5 tyrosine phosphorylation, kinase upregulation, and neurite outgrowth. Neuron 26, 633–646 (2000).
Sasaki, Y. et al. Fyn and Cdk5 mediate semaphorin-3A signaling, which is involved in regulation of dendrite orientation in cerebral cortex. Neuron 35, 907–920 (2002).
Fu, A.K. et al. Cdk5 is involved in neuregulin-induced AChR expression at the neuromuscular junction. Nat. Neurosci. 4, 374–381 (2001).
Yamaguchi, Y. & Pasquale, E.B. Eph receptors in the adult brain. Curr. Opin. Neurobiol. 14, 288–296 (2004).
Flanagan, J.G. & Vanderhaeghen, P. The ephrins and Eph receptors in neural development. Annu. Rev. Neurosci. 21, 309–345 (1998).
Ching, Y.P., Qi, Z. & Wang, J.H. Cloning of three novel neuronal Cdk5 activator binding proteins. Gene 242, 285–294 (2000).
Cheng, K. & Ip, N.Y. Cdk5: a new player at synapses. Neurosignals 12, 180–190 (2003).
Knoll, B. & Drescher, U. Src family kinases are involved in EphA receptor-mediated retinal axon guidance. J. Neurosci. 24, 6248–6257 (2004).
Sahin, M. et al. Eph-dependent tyrosine phosphorylation of ephexin1 modulates growth cone collapse. Neuron 46, 191–204 (2005).
Cowan, C.W. et al. Vav family GEFs link activated Ephs to endocytosis and axon guidance. Neuron 46, 205–217 (2005).
Tolias, K.F. et al. The Rac1-GEF Tiam1 couples the NMDA receptor to the activity-dependent development of dendritic arbors and spines. Neuron 45, 525–538 (2005).
Cheng, Q. et al. Cdk5/p35 and Rho-kinase mediate ephrin-A5-induced signaling in retinal ganglion cells. Mol. Cell. Neurosci. 24, 632–645 (2003).
Egea, J. et al. Regulation of EphA 4 kinase activity is required for a subset of axon guidance decisions suggesting a key role for receptor clustering in Eph function. Neuron 47, 515–528 (2005).
Ehlers, M.D. Activity level controls postsynaptic composition and signaling via the ubiquitin-proteasome system. Nat. Neurosci. 6, 231–242 (2003).
Lai, K.O., Ip, F.C., Cheung, J., Fu, A.K. & Ip, N.Y. Expression of Eph receptors in skeletal muscle and their localization at the neuromuscular junction. Mol. Cell. Neurosci. 17, 1034–1047 (2001).
Hall, A. Rho GTPases and the actin cytoskeleton. Science 279, 509–514 (1998).
Shamah, S.M. et al. EphA receptors regulate growth cone dynamics through the novel guanine nucleotide exchange factor ephexin. Cell 105, 233–244 (2001).
Dhavan, R. & Tsai, L.H. A decade of CDK5. Nat. Rev. Mol. Cell Biol. 2, 749–759 (2001).
Cheung, Z.H., Fu, A.K.Y. & Ip, N.Y. Synaptic roles of Cdk5: implications in higher cognitive functions and neurodegenerative diseases. Neuron 50, 13–18 (2006).
Fu, W.Y., Fu, A.K., Lok, K.C., Ip, F.C. & Ip, N.Y. Induction of Cdk5 activity in rat skeletal muscle after nerve injury. Neuroreport 13, 243–247 (2002).
Fu, A.K. et al. Aberrant motor axon projection, acetylcholine receptor clustering, and neurotransmission in cyclin-dependent kinase 5 null mice. Proc. Natl. Acad. Sci. USA 102, 15224–15229 (2005).
Yuste, R. & Bonhoeffer, T. Genesis of dendritic spines: insights from ultrastructural and imaging studies. Nat. Rev. Neurosci. 5, 24–34 (2004).
Bryan, B. et al. GEFT, a Rho family guanine nucleotide exchange factor, regulates neurite outgrowth and dendritic spine formation. J. Biol. Chem. 279, 45824–45832 (2004).
Ryan, X.P. et al. The Rho-specific GEF Lfc interacts with neurabin and spinophilin to regulate dendritic spine morphology. Neuron 47, 85–100 (2005).
Zhang, H., Webb, D.J., Asmussen, H., Niu, S. & Horwitz, A.F.A. GIT1/PIX/Rac/PAK signaling module regulates spine morphogenesis and synapse formation through MLC. J. Neurosci. 25, 3379–3388 (2005).
Ohshima, T. et al. Impairment of hippocampal long-term depression and defective spatial learning and memory in p35 mice. J. Neurochem. 94, 917–925 (2005).
Kennedy, M.B., Beale, H.C., Carlisle, H.J. & Washburn, L.R. Integration of biochemical signalling in spines. Nat. Rev. Neurosci. 6, 423–434 (2005).
Muller, D., Toni, N. & Buchs, P.A. Spine changes associated with long-term potentiation. Hippocampus 10, 596–604 (2000).
Toni, N., Buchs, P.A., Nikonenko, I., Bron, C.R. & Muller, D. LTP promotes formation of multiple spine synapses between a single axon terminal and a dendrite. Nature 402, 421–425 (1999).
Engert, F. & Bonhoeffer, T. Dendritic spine changes associated with hippocampal long-term synaptic plasticity. Nature 399, 66–70 (1999).
Zhou, Q., Homma, K.J. & Poo, M.M. Shrinkage of dendritic spines associated with long-term depression of hippocampal synapses. Neuron 44, 749–757 (2004).
Nagerl, U.V., Eberhorn, N., Cambridge, S.B. & Bonhoeffer, T. Bidirectional activity-dependent morphological plasticity in hippocampal neurons. Neuron 44, 759–767 (2004).
Gerlai, R. et al. Regulation of learning by EphA receptors: a protein targeting study. J. Neurosci. 19, 9538–9549 (1999).
Ip, N.Y., Li, Y., Yancopoulos, G.D. & Lindsay, R.M. Cultured hippocampal neurons show responses to BDNF, NT-3, and NT-4, but not NGF. J. Neurosci. 13, 3394–3405 (1993).
Sala, C. et al. Regulation of dendritic spine morphology and synaptic function by Shank and Homer. Neuron 31, 115–130 (2001).
Acknowledgements
We are grateful to A. Kulkarni and T. Curran for Cdk5-null mice, L.H. Tsai for p35-null mice, S. Hisanaga for the recombinant Cdk5-p35 protein, D. Lo for the YFP construct and W. Chau, B. Butt, C. Kwong, K. Hung, K. Ho, W.W.Y. Chien and K. Gong for excellent technical assistance. The expert advice of D. Lo and P. Greer is gratefully acknowledged. We also thank Z. Cheung and M. Zhang for critical reading of the manuscript and members of the Ip laboratory for many helpful discussions. This study was supported in part by the Research Grants Council of Hong Kong (HKUST6131/02M, 6130/03M, 6119/04M, 6421/05M and HKUST 3/03C), the Area of Excellence Scheme of the University Grants Committee (AoE/B-15/01), the Hong Kong Jockey Club, US National Institute of Child Health and Human Development grant K08 HD01384, the William Randolph Hearst Fund (to M.S.), Mental Retardation Research Center grant HD18655, and US National Institutes of Health grant NS045500 (to M.E.G.). N.Y.I. is a Croucher Foundation Senior Research Fellow.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
EphA4 interacts with Cdk5 and p35. (PDF 478 kb)
Supplementary Fig. 2
Cdk5 is directly phosphorylated by EphA4. (PDF 379 kb)
Supplementary Fig. 3
Ephrin-A1 stimulates EphA4 clustering in cultured hippocampal neurons. (PDF 2246 kb)
Supplementary Fig. 4
Presence of EphA4, Cdk5.p35 and ephexin1 in postsynaptic densities. (PDF 472 kb)
Supplementary Fig. 5
Ephrin-A1 reduces spine density in cultured hippocampal neurons. (PDF 1864 kb)
Supplementary Fig. 6
Ephexin1 protein is phosphorylated by recombinant Cdk5.p35 using in vitro kinase assay. (PDF 574 kb)
Supplementary Fig. 7
Confocal images showing the expression of overexpressed ephexin1 in hippocampal slices. (PDF 1188 kb)
Supplementary Fig. 8
Model of regulation of spine morphogenesis by EphA4-Cdk5-ephexin1 signaling. (PDF 4228 kb)
Rights and permissions
About this article
Cite this article
Fu, WY., Chen, Y., Sahin, M. et al. Cdk5 regulates EphA4-mediated dendritic spine retraction through an ephexin1-dependent mechanism. Nat Neurosci 10, 67–76 (2007). https://doi.org/10.1038/nn1811
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn1811
This article is cited by
-
Activation of multiple Eph receptors on neuronal membranes correlates with the onset of optic neuropathy
Eye and Vision (2023)
-
Interactions between EGFR and EphA2 promote tumorigenesis through the action of Ephexin1
Cell Death & Disease (2022)
-
Akt-mediated Ephexin1–Ras interaction promotes oncogenic Ras signaling and colorectal and lung cancer cell proliferation
Cell Death & Disease (2021)
-
p39-associated Cdk5 activity regulates dendritic morphogenesis
Scientific Reports (2020)
-
EphA4 loss improves social memory performance and alters dendritic spine morphology without changes in amyloid pathology in a mouse model of Alzheimer’s disease
Alzheimer's Research & Therapy (2019)