Abstract
Episodic memories formed during the first postnatal period are rapidly forgotten, a phenomenon known as 'infantile amnesia'. In spite of this memory loss, early experiences influence adult behavior, raising the question of which mechanisms underlie infantile memories and amnesia. Here we show that in rats an experience learned during the infantile amnesia period is stored as a latent memory trace for a long time; indeed, a later reminder reinstates a robust, context-specific and long-lasting memory. The formation and storage of this latent memory requires the hippocampus, follows a sharp temporal boundary and occurs through mechanisms typical of developmental critical periods, including the expression switch of the NMDA receptor subunits from 2B to 2A, which is dependent on brain-derived neurotrophic factor (BDNF) and metabotropic glutamate receptor 5 (mGluR5). Activating BDNF or mGluR5 after training rescues the infantile amnesia. Thus, early episodic memories are not lost but remain stored long term. These data suggest that the hippocampus undergoes a developmental critical period to become functionally competent.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Change history
29 August 2016
In the version of this article initially published, y-axis labels in Figures 3a,b, 4c, 5d and 6c report the “GluN2B/GluN2A ratio”; this should be changed to “GluN2A/GluN2B ratio.” The error has been corrected in the HTML and PDF versions of the article.
01 July 2017
Nat. Neurosci. 19, 1225–1233 (2016); published online 18 July 2016; corrected after print 29 August 2016 In the version of this article initially published, y-axis labels in Figures 3a,b, 4c, 5d and 6c report the “GluN2B/GluN2A ratio”; this should be “GluN2A/GluN2B ratio.” The error has been corrected in the HTML and PDF versions of the article.
References
Campbell, B.A. & Spear, N.E. Ontogeny of memory. Psychol. Rev. 79, 215–236 (1972).
Hayne, H. Infant memory development: implications for childhood amnesia. Dev. Rev. 24, 33–73 (2004).
Rovee-Collier, C. The development of infant memory. Curr. Dir. Psychol. Sci. 8, 80–85 (1999).
Heim, C. & Nemeroff, C.B. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol. Psychiatry 49, 1023–1039 (2001).
Dumas, T.C. & Rudy, J.W. Development of the hippocampal memory system: creating networks and modifiable synapses. in Oxford Handbook of Developmental Behavioral Neuroscience (eds. Blumberg, M.S., Freeman, J.H. & Robinson, S.R.) (Oxford, 2010).
Nelson, C.A. Neural plasticity in human development: the role of early experience in sculpting memory systems. Dev. Sci. 3, 115–136 (2000).
Akers, K.G. et al. Hippocampal neurogenesis regulates forgetting during adulthood and infancy. Science 344, 598–602 (2014).
Callaghan, B.L., Li, S. & Richardson, R. The elusive engram: what can infantile amnesia tell us about memory? Trends Neurosci. 37, 47–53 (2014).
Kim, J.H., McNally, G. & Richardson, R. Recovery of fear memories in rats: role of gamma-amino butyric acid (GABA) in infantile amnesia. Behav. Neurosci. 120, 40–48 (2006).
Campbell, B.A. & Jaynes, J. Reinstatement. Psychol. Rev. 73, 478–480 (1966).
Spear, N.E. & Parsons, P.J. Analysis of a reactivation treatment: ontogenetic determinants of alleviated forgetting. in Processes in Animal Memory (eds. Medin, D.L., Roberts, W.A. & Davis, R.T.) 135–165 (Lawrence Erlbaum Associates, 1976).
Haroutunian, V. & Riccio, D.C. Effect of arousal conditions during reinstatement treatment upon learned fear in young rats. Dev. Psychobiol. 10, 25–32 (1977).
Davis, J.M. & Rovee-Collier, C.K. Alleviated forgetting of a learned contingency in 8-week-old infants. Dev. Psychol. 19, 353–365 (1983).
Campbell, B.A. & Campbell, E.H. Retention and extinction of learned fear in infant and adult rats. J. Comp. Physiol. Psychol. 55, 1–8 (1962).
Rudy, J.W. & Morledge, P. Ontogeny of contextual fear conditioning in rats: implications for consolidation, infantile amnesia, and hippocampal system function. Behav. Neurosci. 108, 227–234 (1994).
Squire, L.R., Wixted, J.T. & Clark, R.E. Recognition memory and the medial temporal lobe: a new perspective. Nat. Rev. Neurosci. 8, 872–883 (2007).
Chen, D.Y., Bambah-Mukku, D., Pollonini, G. & Alberini, C.M. Glucocorticoid receptors recruit the CaMKIIα-BDNF-CREB pathways to mediate memory consolidation. Nat. Neurosci. 15, 1707–1714 (2012).
Raineki, C. et al. Functional emergence of the hippocampus in context fear learning in infant rats. Hippocampus 20, 1037–1046 (2010).
Foster, J.A. & Burman, M.A. Evidence for hippocampus-dependent contextual learning at postnatal day 17 in the rat. Learn. Mem. 17, 259–266 (2010).
Robinson-Drummer, P.A. & Stanton, M.E. Using the context preexposure facilitation effect to study long-term context memory in preweanling, juvenile, adolescent, and adult rats. Physiol. Behav. 148, 22–28 (2015).
Minichiello, L. TrkB signaling pathways in LTP and learning. Nat. Rev. Neurosci. 10, 850–860 (2009).
Morris, R.G., Anderson, E., Lynch, G.S. & Baudry, M. Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5. Nature 319, 774–776 (1986).
Tsien, J.Z., Huerta, P.T. & Tonegawa, S. The essential role of hippocampal CA1 NMDA receptor-dependent synaptic plasticity in spatial memory. Cell 87, 1327–1338 (1996).
Constantine-Paton, M., Cline, H.T. & Debski, E. Patterned activity, synaptic convergence, and the NMDA receptor in developing visual pathways. Annu. Rev. Neurosci. 13, 129–154 (1990).
Sans, N. et al. A developmental change in NMDA receptor-associated proteins at hippocampal synapses. J. Neurosci. 20, 1260–1271 (2000).
Paoletti, P., Bellone, C. & Zhou, Q. NMDA receptor subunit diversity: impact on receptor properties, synaptic plasticity and disease. Nat. Rev. Neurosci. 14, 383–400 (2013).
Hensch, T.K. Critical period plasticity in local cortical circuits. Nat. Rev. Neurosci. 6, 877–888 (2005).
Bellone, C. & Nicoll, R.A. Rapid bidirectional switching of synaptic NMDA receptors. Neuron 55, 779–785 (2007).
Carmignoto, G. & Vicini, S. Activity-dependent decrease in NMDA receptor responses during development of the visual cortex. Science 258, 1007–1011 (1992).
Quinlan, E.M., Philpot, B.D., Huganir, R.L. & Bear, M.F. Rapid, experience-dependent expression of synaptic NMDA receptors in visual cortex in vivo. Nat. Neurosci. 2, 352–357 (1999).
Philpot, B.D., Sekhar, A.K., Shouval, H.Z. & Bear, M.F. Visual experience and deprivation bidirectionally modify the composition and function of NMDA receptors in visual cortex. Neuron 29, 157–169 (2001).
Caldeira, M.V. et al. BDNF regulates the expression and traffic of NMDA receptors in cultured hippocampal neurons. Mol. Cell. Neurosci. 35, 208–219 (2007).
Liu, L. et al. Role of NMDA receptor subtypes in governing the direction of hippocampal synaptic plasticity. Science 304, 1021–1024 (2004).
Yashiro, K. & Philpot, B.D. Regulation of NMDA receptor subunit expression and its implications for LTD, LTP, and metaplasticity. Neuropharmacology 55, 1081–1094 (2008).
Matta, J.A., Ashby, M.C., Sanz-Clemente, A., Roche, K.W. & Isaac, J.T. mGluR5 and NMDA receptors drive the experience- and activity-dependent NMDA receptor NR2B to NR2A subunit switch. Neuron 70, 339–351 (2011).
Huang, Z.J. et al. BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell 98, 739–755 (1999).
Gianfranceschi, L. et al. Visual cortex is rescued from the effects of dark rearing by overexpression of BDNF. Proc. Natl. Acad. Sci. USA 100, 12486–12491 (2003).
Schulenburg, C.J., Riccio, D.C. & Stikes, E.R. Acquisition and retention of a passive-avoidance response as a function of age in rats. J. Comp. Physiol. Psychol. 74, 75–83 (1971).
Balsam, P.D., Drew, M.R. & Gallistel, C.R. Time and associative learning. Comp. Cogn. Behav. Rev. 5, 1–22 (2010).
Cull-Candy, S., Brickley, S. & Farrant, M. NMDA receptor subunits: diversity, development and disease. Curr. Opin. Neurobiol. 11, 327–335 (2001).
Gambrill, A.C. & Barria, A. NMDA receptor subunit composition controls synaptogenesis and synapse stabilization. Proc. Natl. Acad. Sci. USA 108, 5855–5860 (2011).
Dumas, T.C. Developmental regulation of cognitive abilities: modified composition of a molecular switch turns on associative learning. Prog. Neurobiol. 76, 189–211 (2005).
Berardi, N., Pizzorusso, T. & Maffei, L. Critical periods during sensory development. Curr. Opin. Neurobiol. 10, 138–145 (2000).
Castrén, M.L. & Castrén, E. BDNF in fragile X syndrome. Neuropharmacology 76, 729–736 (2014).
Greenough, W.T., Black, J.E. & Wallace, C.S. Experience and brain development. Child Dev. 58, 539–559 (1987).
Nelson, C.A. III et al. Cognitive recovery in socially deprived young children: the Bucharest Early Intervention Project. Science 318, 1937–1940 (2007).
Anand, K.J., Coskun, V., Thrivikraman, K.V., Nemeroff, C.B. & Plotsky, P.M. Long-term behavioral effects of repetitive pain in neonatal rat pups. Physiol. Behav. 66, 627–637 (1999).
Zhao, M.G. et al. Roles of NMDA NR2B subtype receptor in prefrontal long-term potentiation and contextual fear memory. Neuron 47, 859–872 (2005).
Qi, C. et al. mGluR5 in the nucleus accumbens shell regulates morphine-associated contextual memory through reactive oxygen species signaling. Addict. Biol. 20, 927–940 (2015).
Rudy, J.W. & Matus-Amat, P. DHPG activation of group 1 mGluRs in BLA enhances fear conditioning. Learn. Mem. 16, 421–425 (2009).
Acknowledgements
We thank G. Pollonini for technical assistance. We thank P. Magistretti, F. Ansermet, K. Weiss, P. Balsam, X. Ye, F. Fiumara and G. Philips for discussions or comments on the manuscript. This work was supported by R01-MH074736 and an Agalma Foundation grant to C.M.A. and R01 NS072359 to R.D.B. A.T. was supported by a fellowship from Agalma Foundation. R.B. was supported by a fellowship from the Swiss National Science Foundation.
Author information
Authors and Affiliations
Contributions
C.M.A. led the design and development of the study and the writing of the manuscript; R.D.B, designed the electrophysiology study; A.T., R.B., E.S.S., R.D.B. and C.M.A. designed experiments and analyzed data; A.T. carried out behavioral experiments and the majority of molecular and pharmacological experiments; R.B. carried out behavioral experiments and contributed to molecular and pharmacological experiments; E.S.S. carried out electrophysiology experiments; and A.T., E.S.S., R.D.B. and C.M.A. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Short- and long-term memory retentions of adult rats.
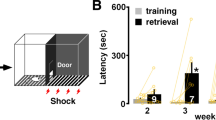
Experimental schedule is shown above each panel. Acquisition (Acq.) and memory retention are expressed as mean latency ± s.e.m. (in seconds, s). (a) Mean latency ± s.e.m. of naïve and rats trained at PN80 (adults) and tested: (a) 30min (T1) and 1d after training (T2) [n= 11, 10, Two–way ANOVA followed by Bonferroni post hoc, Treatment F(1,38)= 98.74, P< 0.0001. Testing F(1,38)= 3.718, P=0.0613, Interaction F(1,38)=3.894, P=0.0558; 3 independent experiments], and (b) 1d (T1) and 7d after training (T2) [n=5, 6, Two–way ANOVA followed by Bonferroni post hoc, Treatment F(1,18)= 121.1, P< 0.0001,Testing F(1,18)= 0.2594, P= 0.6167, Interaction F(1,18)= 0.2921, P= 0.5955; 2 independent experiments]. ***P < 0.001. Latency score in Supplementary Table 10.
Supplementary Figure 2 Nociception of naive PN17 and PN24 rats.
Escape latency is expressed as mean ± s.e.m. (in seconds, s). Pain threshold in infant rats was measured by the escape latency to withdrawal from a 53 ± 1°C hotplate. The escape latency was averaged from four sessions, with an inter-session interval of 15-min. Student’s t-test revealed no significant difference between PN17 (10.1±0.8 seconds, n=6) and PN24 rats (8.2±0.9 seconds, n=6) (two-tailed t test, t =1.562, df=10, P=0.7028).
Supplementary Figure 3 Effect of training or shock-only on rat weight gain.
Gain weight compared to the weight taken immediately before training is expressed as mean ± s.e.m. (in grams, g). Training and shock-only at (a) PN17 [n= 8, 8, 8, Two–way ANOVA followed by Bonferroni post hoc, Treatment F(2,42)= 1.332, P>0.05, Testing F(1,42)= 2515, P<0.0001, Interaction F(1,42)= 1.656, P>0.05; 3 independent experiments] or (b) PN24 [n= 8, 8, 8, Two–way ANOVA followed by Bonferroni post hoc, Treatment F(2,42)= 0.4406, P>0.05, Testing F(1,42)= 801.7, P<0.0001, Interaction F(1,42)= 0.3349, P>0.05; 3 independent experiments] did not alter the average gain weight measured 1 d and 7 d later. Numeric values in Supplementary Table 11.
Supplementary Figure 4 The unpaired context–shock protocol failed to reinstate memory.
Experimental schedule is shown above the panel. Acquisition (Acq.) and memory retention are expressed as mean latency ± s.e.m. (in seconds, s). In the unpaired protocol, rats were exposed to the IA context similarly to the trained rats but did not receive a footshock in the dark chamber. They were returned to their home cage and, one hour later, were placed directly onto the grid floor of the dark chamber and immediately shocked (1.0 mA). The unpaired protocol failed to reinstate memory (n=8, 8, Two–way ANOVA followed by Bonferroni post hoc, Condition F(1.56,)=1.901, P=0.1735, Testing F(3,56)=4.720, P=0.0053, Interaction F(3,56)=1.245, P=0.3021; 3 independent experiments). Latency score in Supplementary Table 12.
Supplementary Figure 5 Memory reinstatement following different time intervals between test and reminder shock.
Experimental schedule is shown above the panel. Acquisition (Acq.) and memory retention are expressed as mean latency ± s.e.m. (in seconds, s). Mean latency ± s.e.m. of naïve, shock-only and rats trained at PN17, tested 7d later (T1) and given a reminder shock (RS) 4h, 1d or 7d after the test (T1). Memory retention was tested 1d (T2) and 7d (T3) after RS, and, 4d later, in a new context (NC) [n= 11, 13, 11, 11, 11, Two–way ANOVA followed by Bonferroni post hoc, Treatment F(4,208)=18.27, P<0.0001, Testing F(3,208)=26.93, P<0.0001, Interaction F(12,208)=4.269, P<0.0001; 3 independent experiments]. Latency score in Supplementary Table 13.
Supplementary Figure 6 Hippocampal molecular changes either in untrained (naïve) conditions or following IA training at PN17 or PN24.
(a) Representative examples and densitometric western blot analyses of dorsal hippocampal total extracts from naïve rats euthanized at PN17, PN24 or PN80 (adult) (n=8, 8, 8). Data are expressed as mean percentage ± s.e.m. of adult naïve rats [One–way ANOVA followed by Newman-Keuls Multiple Comparison Test, TrkB F(2,21)= 0.6080, P=0.5537; GluN1 F(2,21)= 0.1954, P=0.8240, 3 independent experiments]. (b-c) Representative examples and densitometric western blot analyses of dHC total extracts from rats trained in IA at (b) PN17 or (c) PN24, and euthanized 30min, 9h, 24h or 48h after training (n=6-10/group). To account for developmental differences, two groups of naïve were used [(b) PN17 and PN19 or (c) PN24 and PN26]. Data are expressed as mean percentage ± s.e.m. of (b) PN17 [n= 8, 6, 10, 7, 6, 6, One–way ANOVA followed by Dunnett's Multiple Comparison Test, TrkB F(3,27)= 0.1618, P= 0.9211; GluN1 F(3,27)= 0.08967, P= 0.9650; 3 independent experiments] or (c) PN24 naïve rats [n=8, 6, 6, 7, 7, 8, One–way ANOVA followed by Dunnett's Multiple Comparison Test, TrkB F(3,23)= 0.06099, P= 0.9798; GluN1 F(3,23)= 0.2067, P= 0.8907; 3 independent experiments]. The numeric values are reported in Supplementary Table 3.
Supplementary Figure 7 Hippocampal molecular changes after shock- or context-only.
Densitometry of western blot analyses of dorsal hippocampal total extracts from rats euthanized 24 hours after (a) receiving a footshock immediately after being placed on a shock grid (shock-only) [n= 7, 7, Unpaired two-tailed Student’s t-test, pTrkB t=0.3263 df=12, P = 0.7498; GluN2A t=0.5967 df=12, P = 0.5618l; GluN2B t=0.1414 df=12, P = 0.8899; 2 independent experiments] or (b) exposed to the IA context without receiving the footshock (context-only) [n= 7, 7, Unpaired two-tailed Student’s t-test, pTrkB t=0.5199 df=12, P = 0.6126; GluN2A t=0.6308 df=12, P = 0.5400; GluN2B t=0.4679 df=12 P = 0.6482; 2 independent experiment]. Data are expressed as mean percentage ± s.e.m. of naive rats euthanized at the matched time point (i.e. PN18). The numeric values are reported in Supplementary Table 14.
Supplementary Figure 8 Hippocampal molecular changes after memory reinstatement.
Experimental schedule is shown above the panel. Densitometric western blot analyses of dorsal hippocampal total extracts obtained from rats trained (Tr) at PN17, 7 days later exposed to the reinstatement protocol [test (T1) and 2d later reminder shock (RS)] and euthanized 30min or 24 hours later. Data are expressed as mean percentage ± s.e.m. of rats trained at PN17, tested (T1) but not exposed to RS and euthanized at the matched time point [n=6/group, One–way ANOVA followed by Newman-Keuls Multiple Comparison Test, pTrkB F(2,17)= 0.27, P=0.77; GluN2A F(2,17)= 0.72, P=0.50; GluN2B F(2,17)= 0.08, P=0.82; 2 independent experiment). The numeric values are reported in Supplementary Table 15.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–10 (PDF 1876 kb)
Rights and permissions
About this article
Cite this article
Travaglia, A., Bisaz, R., Sweet, E. et al. Infantile amnesia reflects a developmental critical period for hippocampal learning. Nat Neurosci 19, 1225–1233 (2016). https://doi.org/10.1038/nn.4348
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.4348
This article is cited by
-
The generative grammar of the brain: a critique of internally generated representations
Nature Reviews Neuroscience (2024)
-
Engram neurons: Encoding, consolidation, retrieval, and forgetting of memory
Molecular Psychiatry (2023)
-
Forgetting as a form of adaptive engram cell plasticity
Nature Reviews Neuroscience (2022)
-
A Baldwin interpretation of adult hippocampal neurogenesis: from functional relevance to physiopathology
Molecular Psychiatry (2022)
-
A critical period for learning and plastic changes at hippocampal CA1 synapses
Scientific Reports (2022)