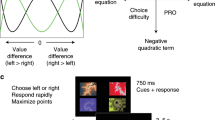
Abstract
Previous theories predict that human dorsal anterior cingulate (dACC) should respond to decision difficulty. An alternative theory has been recently advanced that proposes that dACC evolved to represent the value of 'non-default', foraging behavior, calling into question its role in choice difficulty. However, this new theory does not take into account that choosing whether or not to pursue foraging-like behavior can also be more difficult than simply resorting to a default. The results of two neuroimaging experiments show that dACC is only associated with foraging value when foraging value is confounded with choice difficulty; when the two are dissociated, dACC engagement is only explained by choice difficulty, and not the value of foraging. In addition to refuting this new theory, our studies help to formalize a fundamental connection between choice difficulty and foraging-like decisions, while also prescribing a solution for a common pitfall in studies of reward-based decision making.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Rushworth, M.F.S., Kolling, N., Sallet, J. & Mars, R.B. Valuation and decision-making in frontal cortex: one or many serial or parallel systems? Curr. Opin. Neurobiol. 22, 946–955 (2012).
Shenhav, A., Botvinick, M.M. & Cohen, J.D. The expected value of control: an integrative theory of anterior cingulate cortex function. Neuron 79, 217–240 (2013).
Alexander, W.H. & Brown, J.W. Medial prefrontal cortex as an action-outcome predictor. Nat. Neurosci. 14, 1338–1344 (2011).
Hare, T.A., Schultz, W., Camerer, C.F., O'Doherty, J.P. & Rangel, A. Transformation of stimulus value signals into motor commands during simple choice. Proc. Natl. Acad. Sci. USA 108, 18120–18125 (2011).
Shackman, A.J. et al. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat. Rev. Neurosci. 12, 154–167 (2011).
Venkatraman, V. & Huettel, S.A. Strategic control in decision-making under uncertainty. Eur. J. Neurosci. 35, 1075–1082 (2012).
Holroyd, C.B. & Yeung, N. Motivation of extended behaviors by anterior cingulate cortex. Trends Cogn. Sci. 16, 122–128 (2012).
Behrens, T.E.J., Woolrich, M.W., Walton, M.E. & Rushworth, M.F.S. Learning the value of information in an uncertain world. Nat. Neurosci. 10, 1214–1221 (2007).
Rushworth, M.F.S., Noonan, MaryAnn P., Boorman, E.D., Walton, M.E. & Behrens, T.E. Frontal cortex and reward-guided learning and decision-making. Neuron 70, 1054–1069 (2011).
Duncan, J. & Owen, A.M. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci. 23, 475–483 (2000).
Paus, T., Koski, L., Caramanos, Z. & Westbury, C. Regional differences in the effects of task difficulty and motor output on blood flow response in the human anterior cingulate cortex: a review of 107 PET activation studies. Neuroreport 9, R37–R47 (1998).
Pochon, J.-B., Riis, J., Sanfey, A.G., Nystrom, L.E. & Cohen, J.D. Functional imaging of decision conflict. J. Neurosci. 28, 3468–3473 (2008).
FitzGerald, T.H.B., Seymour, B. & Dolan, R.J. The role of human orbitofrontal cortex in value comparison for incommensurable objects. J. Neurosci. 29, 8388–8395 (2009).
Botvinick, M.M., Braver, T.S., Barch, D.M., Carter, C.S. & Cohen, J.D. Conflict monitoring and cognitive control. Psychol. Rev. 108, 624–652 (2001).
Botvinick, M.M. Conflict monitoring and decision making: reconciling two perspectives on anterior cingulate function. Cogn. Affect. Behav. Neurosci. 7, 356–366 (2007).
Shenhav, A. & Buckner, R.L. Neural correlates of dueling affective reactions to win-win choices. Proc. Natl. Acad. Sci. USA 10.1073/pnas.1405725111 (14 July 2014).
Kolling, N., Behrens, T.E.J., Mars, R.B. & Rushworth, M.F.S. Neural mechanisms of foraging. Science 336, 95–98 (2012).
Pearson, J.M., Watson, K.K. & Platt, M.L. Decision making: the neuroethological turn. Neuron 82, 950–965 (2014).
Boorman, E.D., Rushworth, M.F. & Behrens, T.E. Ventromedial prefrontal and anterior cingulate cortex adopt choice and default reference frames during sequential multi-alternative choice. J. Neurosci. 33, 2242–2253 (2013).
Kolling, N., Wittmann, M. & Rushworth, M.F.S. Multiple neural mechanisms of decision making and their competition under changing risk pressure. Neuron 81, 1190–1202 (2014).
Mobbs, D. et al. Foraging under competition: the neural basis of input-matching in humans. J. Neurosci. 33, 9866–9872 (2013).
Hayden, B.Y., Pearson, J.M. & Platt, M.L. Neuronal basis of sequential foraging decisions in a patchy environment. Nat. Neurosci. 14, 933–939 (2011).
Charnov, E.L. Optimal foraging, the marginal value theorem. Theor. Popul. Biol. 9, 129–136 (1976).
Stephens, D.W. & Krebs, J.R. Foraging Theory (Princeton Univ. Press, 1986).
Ratcliff, R. A theory of memory retrieval. Psychol. Rev. 85, 59–108 (1978).
Bogacz, R., Brown, E., Moehlis, J., Holmes, P. & Cohen, J.D. The physics of optimal decision making: a formal analysis of models of performance in two-alternative forced-choice tasks. Psychol. Rev. 113, 700–765 (2006).
Milosavljevic, M., Malmaud, J., Huth, A., Koch, C. & Rangel, A. The Drift Diffusion Model can account for the accuracy and reaction time of value-based choices under high and low time pressure. Judgm. Decis. Mak. 5, 437–449 (2010).
Krajbich, I., Armel, C. & Rangel, A. Visual fixations and the computation and comparison of value in simple choice. Nat. Neurosci. 13, 1292–1298 (2010).
Basten, U., Biele, G., Heekeren, H.R. & Fiebach, C.J. How the brain integrates costs and benefits during decision making. Proc. Natl. Acad. Sci. USA 107, 21767–21772 (2010).
Grinband, J. et al. The dorsal medial frontal cortex is sensitive to time on task, not response conflict or error likelihood. Neuroimage 57, 303–311 (2011).
Weissman, D.H. & Carp, J. The congruency effect in the posterior medial frontal cortex is more consistent with time on task than with response conflict. PLoS ONE 8, e62405 (2013).
Wessel, J.R., Danielmeier, C., Morton, J.B. & Ullsperger, M. Surprise and error: common neuronal architecture for the processing of errors and novelty. J. Neurosci. 32, 7528–7537 (2012).
Garrison, J., Erdeniz, B. & Done, J. Prediction error in reinforcement learning: a meta-analysis of neuroimaging studies. Neurosci. Biobehav. Rev. 37, 1297–1310 (2013).
Hayden, B.Y., Heilbronner, S.R., Pearson, J.M. & Platt, M.L. Surprise signals in anterior cingulate cortex: neuronal encoding of unsigned reward prediction errors driving adjustment in behavior. J. Neurosci. 31, 4178–4187 (2011).
Bryden, D.W., Johnson, E.E., Tobia, S.C., Kashtelyan, V. & Roesch, M.R. Attention for learning signals in anterior cingulate cortex. J. Neurosci. 31, 18266–18274 (2011).
Cavanagh, J.F. & Frank, M.J. Frontal theta as a mechanism for cognitive control. Trends Cogn. Sci. 10.1016/j.tics.2014.04.012 (15 May 2014).
Kriegeskorte, N., Simmons, W.K., Bellgowan, P.S.F. & Baker, C.I. Circular analysis in systems neuroscience: the dangers of double dipping. Nat. Neurosci. 12, 535–540 (2009).
Yeung, N., Botvinick, M.M. & Cohen, J.D. The neural basis of error detection: conflict monitoring and the error-related negativity. Psychol. Rev. 111, 931–959 (2004).
Brainard, D.H. The Psychophysics Toolbox. Spat. Vis. 10, 433–436 (1997).
Mulder, M.J., Wagenmakers, E.-J., Ratcliff, R., Boekel, W. & Forstmann, B.U. Bias in the brain: a diffusion model analysis of prior probability and potential payoff. J. Neurosci. 32, 2335–2343 (2012).
Penny, W., Kiebel, S. & Friston, K. Variational Bayesian inference for fMRI time series. Neuroimage 19, 727–741 (2003).
Stephan, K.E., Penny, W.D., Daunizeau, J., Moran, R.J. & Friston, K.J. Bayesian model selection for group studies. Neuroimage 46, 1004–1017 (2009).
Hare, T.A., O'Doherty, J.P., Camerer, C., Schultz, W. & Rangel, A. Dissociating the role of the orbitofrontal cortex and the striatum in the computation of goal values and prediction errors. J. Neurosci. 28, 5623–5630 (2008).
Acknowledgements
The authors are grateful to S. Feng for assistance in data analysis. This work is supported by the C.V. Starr Foundation (A.S.), the National Institute of Mental Health R01MH098815-01 (M.M.B.) and the John Templeton Foundation. The opinions expressed in this publication are those of the authors and do not necessarily reflect the views of the John Templeton Foundation.
Author information
Authors and Affiliations
Contributions
A.S., J.D.C. and M.M.B. designed the experiments; A.S. and M.A.S. performed the experiments; A.S. analyzed the data; and A.S., J.D.C. and M.M.B. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Behavior across the two experiments
a–b) Average beta estimates from a logistic regression for (A) Stage 1 of the task (Foraging Phase), predicting choices to forage and (b) Stage 2 of the task (Engage Phase), predicting choices of a leftward response (error bars, s.e.m.). Upper insets reproduce the regression estimates from KBMR’s experiment. From Kolling, N., Behrens, T.E.J., Mars, R.B. & Rushworth, M.F.S. Neural mechanisms of foraging. Science 336, 95-98 (2012). Reprinted with permission from AAAS. c) Histogram of forage choice proportions across participants. Blue bars show distributions of actual choice averages; green bars show the simulated histograms if participants had chosen exclusively based on RVforage. The two histograms are shown overlapping rather than stacked. While one participant in Exp 2 showed a strong bias toward foraging, we chose not to exclude this individual for two reasons. First, unlike insufficient foraging (KBMR’s exclusion criterion; see Online Methods), a bias to forage posed no risk of under-powering our analyses. Second, in their original paper as well as a more recent one20, KBMR’s analyses assume that the default status of an option (in this case, the Engage option) is fixed across participants, including those who evince no bias or a reversed bias (which they term greater “foraging readiness”). Moreover, the participants at this extreme (least biased toward the default) are predicted to have the strongest dACC responses to foraging value. We address the logic of this prediction in the legend to Supplementary Fig. 7 but for this reason felt it important not to exclude this participant based on over-foraging. Instead, we simply note that all of the qualitative results hold when excluding this participant, and include scatterplots in Supplementary Figs. 3, 4, and 8 to emphasize that none of our key findings were driven by potential outliers. d) Averages and ranges of RTs (log-transformed) across subjects for each phase of the two experiments. * Signed-rank P < 0.05, ** Signed-rank P < 0.01.
Supplementary Figure 2 Modeling of choice behavior for the foraging task
The DDM was fit to choice and RT data from each stage of the task for the two experiments, with drift rate defined by the relative value (based on the explicit values shown for a given trial) plus an additional offset term that was allowed to vary during the fitting procedure. While each participant’s data were fit individually, for visualization purposes solid black curves show the means and s.e.m. for a fixed-effects analysis across the group (binning relative values) and solid red curves show the predicted choice probability and RT based on the best-fit DDM parameters to the group data. Histograms show the frequency of trials within each forage value bin. Black vertical dashed lines show the indifference point predicted by explicit reward values alone and red vertical dashed lines show the indifference point defined by the best-fit offset point. In Stage 2, these two overlap because participants are not significantly biased toward one option. Conversely, in Stage 1 the offset term diverges from the point of greatest value similarity due to an engage-favoring bias. The relative value range sampled most densely in Exp 1 correlated with increasing choice difficulty (left), consistent with the linear relationship also observed with RT (right).
Supplementary Figure 3 Experiment 2 shows that the dACC foraging value effect is highly sensitive to the range of foraging values tested
a) We tested our dACC ROI for a linear effect of foraging value across a sliding window of foraging value bins (spanning 70% of trials at a time). We found significant effects in the earliest windows (most comparable to Exp 1 and KBMR’s original study) but not the later ones. b) A closer examination (using windows of 50% of trials at a time) shows the reversal of the foraging value effect from a significant positive effect (20–70% window) to a significant negative effect (50–100% window). See also Fig. 5c and Supplementary Fig. 8d,f. c) Scatterplots of data from key windows in panels a and b show that both the positive and negative effects of foraging value appear in almost all our participants. Error bars reflect s.e.m. * P < 0.05, *** P < 0.005.
Supplementary Figure 4 Results from experiment 2, showing that choice difficulty provides a better model of dACC responses than foraging value
a) Scatter plot showing all beta estimates for foraging value and choice difficulty in Exp 2. Each pair of colored dots represents a single participant (green: foraging value; red: choice difficulty). Beta estimates are taken from a simultaneous regression (GLM 4) that also included a regressor for foraging costs. b) Whole-brain t-statistic map for the contrast between the difficulty and foraging value regressors in the same GLM, thresholded at voxelwise P < 0.01, extent threshold of 200 voxels. c–d) Bayesian model comparisons shows that choice difficulty is a more likely model than foraging value for Exp 2 data within dACC, as indicated by relative exceedance probabilities (c) across the whole brain, using a comparison only of foraging value and difficulty models (unthresholded; activations in red show regions where the choice difficulty model has an exceedance probability greater than the foraging value model) and (d) averaged across voxels within our a priori defined ROI, comparing foraging value, choice difficulty, and a “baseline” model that simply omits any parametric modulation of foraging trials.
Supplementary Figure 5 Different measures show dACC engagement reflecting foraging choice difficulty
a) Choice difficulty is estimated by the similarity of foraging values, corrected for individual engage biases (same as in Figs. 3c, top and 5b,d). b) Choice difficulty is estimated by the difference between DDM-projected choice probabilities for a given trial and indifference (50%; see “Behavioral analysis” section of Online Methods). c) Choice difficulty is estimated by the difference between logistic regression estimated choice probabilities and indifference. Aside from being based on a simpler model, note that these estimates differ in a few ways from those generated by the DDM in panel c. First, they are based on regression fits to all possible relevant choice values on a trial rather than a single relative value measure. Second, they are based only on fits to choice data rather than both choice and RT data.
Supplementary Figure 6 Choice difficulty accounts for other findings that have been argued to reflect evaluation of the non-default option in dACC: example 1
Boorman and colleagues19 had participants choose among three items based on the magnitude of potential reward and the probability of winning that reward (see their Fig. 1). a) Top: The authors found that choices were sensitive to the overall expected value (EV), but that participants treated the item with the highest probability as the default (i.e., EVs being equal, participants were biased to select the highest-probability item). Bottom: Boorman and colleagues also found that dACC activity decreased as the EV of the default (highest-probability) item increased (Default V1), and increased as the EV of the other two options increased (Default V2-V3). Both panels adapted, with permission, from Boorman, E.D., Rushworth, M.F. & Behrens, T.E. Ventromedial prefrontal and anterior cingulate cortex adopt choice and default reference frames during sequential multi-alternative choice. J. Neurosci. 33, 2242-2253 (2013). b) Top: We simulated decision-makers who chose based on the relevant decision variables (reward, probability, EV), but with an additional bias toward the highest-probability item, and obtain comparable behavior (see Online Methods). Bottom: Consistent with the observed patterns of dACC activity, we also show that these decision-makers will experience less choice difficulty (i.e., entropy) as the value of their default increases, but that difficulty will increase with the value of either alternative (right). Error bars reflect s.e.m.
Supplementary Figure 7 Choice difficulty accounts for other findings that have been argued to reflect evaluation of the non-default option in dACC: example 2
a) Kolling and colleagues20 had participants make risky choices (reward shown numerically, probabilities by height of blue bar) in order to reach a cumulative reward target for a given block of trials (the cumulative reward, target, and number of trials remaining are all shown). One of these options was always riskier (lower probability gamble) than the other. b) Participants were sensitive to a measure of the relative expected values between the two options (RVrisky) and to a measure of the distance between their current reward level and the current target (risk pressure). They also displayed a clear bias toward the safer option (seen in the logistic regression values and in the overall displacement of the choice curve to below the 50% line), suggesting that both RVrisky and risk pressure marked the value of the non-default (risky) option. We simulate this scenario, producing qualitatively similar behavioral effects (blue points; error bars, s.e.m.; means for original data reproduced in black). c) The authors report that dACC activity was greater when choosing risky relative to safe choices [left], increased with RVrisky (across trials) [middle], and increased with risk pressure, moreso when choosing the safe option than when choosing the risky option [right]. Panels a, c, and black markers in b adapted from Kolling, N., Wittmann, M. & Rushworth, M.F.S. Multiple neural mechanisms of decision making and their competition under changing risk pressure. Neuron 81, 1190-1202 (2014), which is licensed under CC BY 3.0 (http://creativecommons.org/licenses/by/3.0/). d) We show that similar effects are obtained when using simulated choice difficulty (absolute choice probability, relative to 100% certainty of one option or another) instead of dACC activity as the dependent variable of interest. The authors report additional findings in dACC (not shown), including one that is not predicted by our simulations. While the authors show an increasing effect of RVrisky in dACC across all trials (Panel c, middle), a subsequent ROI analysis attempts to show that this effect was greater when subjects chose the risky rather than the safe option (the opposite of the results predicted and obtained for risk pressure [Panel c, right]). The RVrisky result runs counter to those predicted by our simulations. However, it should be noted that, unlike the whole-brain analyses (and unlike the ROI analyses reported by Boorman and colleagues19), the analysis that produced the result suffers from, and may be partially explained by, selection bias (see Supplementary Fig. 8). More generally, it remains unclear why a foraging account would at the same time predict similar17, greater20, and smaller20 dACC foraging value effects when choosing the non-default versus the default. Similar ambiguity exists with respect to predicted individual differences within this and KBMR’s original study, and whether they are limited to only certain parameters of the foraging decision and/or a subset of choices, and whether individual differences in foraging bias should be taken as a measure of “default”-ness or whether one option can be labeled a default irrespective of behavior (interpretations in both papers strongly suggest the latter, given the expectation that some of these participants revealed no behavioral bias or a reversed bias and yet they were predicted to show the strongest neural representation of foraging value). These considerations all bear on the dispositive value of a given finding as additional unique support for a foraging account, relative to a more context-general account.
Supplementary Figure 8 Correlations between dACC activity and forage value, by forage choice
One of KBMR’s results that do not fit intuitively with a choice difficulty account is the finding of a foraging value effect in a dACC ROI on trials where participants chose to ‘forage’ rather than ‘engage.’ In order to scrutinize the effect more closely, we tested for it in our own data. a–d) Whole-brain analyses identify a large cluster of voxels in dACC positively correlated with foraging value (RVforage) on Choose Engage trials in Exp 1–2 (a,c), as in the original study. On Choose Forage trials we find no such correlation in Exp 1(b), and instead see that the correlation reverses in Exp 2 (panel d, regions in blue-green). All maps are shown at p<0.01, no extent threshold. e) Focusing on our main dACC ROI (left panel), we found a strong foraging value effect on Choose Engage trials (t14 = 6.0, P = 3.3 x 10–5, signed-rank P = 2.0 x 10–4), and on Choose Forage trials identified a marginally significant effect in the same direction (t14 = 1.8, P = 0.10, signed-rank P = 0.07). This may suggest that we were simply under-powered to identify the latter effect, but further analyses suggest a deeper issue. Note first that the effects just described were identified within voxels selected on the basis of showing the strongest group foraging value effect within the same study (an ROI which we otherwise only use to make inferences regarding Exp 2 data). While mirroring KBMR’s analytic approach (see also Figs. 4Di and 5A in Ref. 20), this non-independent selection approach biases the analysis toward identifying a positive correlation in any subset of trials based in part on noise36. We therefore performed the equivalent analysis using a cross-validation approach – selecting an ROI based on the peak (linear) foraging value effect in Exp 2 (right panel; see Supplementary Fig. 3b). We were able to again show a robust foraging value effect on Choose Engage trials (t14 = 4.9, P = 2.3 x 10–4, signed-rank P = 8.5 x 10–4), but the effect is now eliminated for Choose Forage trials (t14= 0.23, P = 0.82, signed-rank P = 0.60; Supplementary Fig. 7E). f) In Exp 2 we tested a wide enough range of foraging values such that a positive foraging value effect on Choose Forage trials would indeed be difficult to explain on the basis of difficulty alone. For this experiment, using a foraging value dACC ROI defined in Exp 1 (as elsewhere in the main text), we again see a significant positive effect of foraging value on dACC activity on Choose Engage trials (t13 = 5.1, P = 2.0 x 10–4, signed-rank P = 1.2 x 10–4) but instead see a significant negative effect of foraging value on Choose Forage trials (t13 = –5.0, P = 2.6 x 10–4, signed-rank P = 8.5 x 10–4), as a choice difficulty account predicts (see also Fig. 2E and Supplementary Fig. 3). This reversal was present for almost every participant (colored lines).
Supplementary Figure 9 A region of rostral dACC tracked the level of surprise participants might have experienced for the foraging value on a given trial
Maps show regions that positively correlated with foraging value surprise, or the degree to which a given RVforage value differed (positively or negatively) from the average observed RVforage value up to that point. In both Exp 1 (top) and Exp 2 (bottom), we found that a region of rostral dACC was among the regions that tracked this measure. Maps are thresholded at P < 0.01, extent threshold of 200 voxels.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–9 (PDF 5775 kb)
Rights and permissions
About this article
Cite this article
Shenhav, A., Straccia, M., Cohen, J. et al. Anterior cingulate engagement in a foraging context reflects choice difficulty, not foraging value. Nat Neurosci 17, 1249–1254 (2014). https://doi.org/10.1038/nn.3771
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.3771
This article is cited by
-
Reward maximization assessed using a sequential patch depletion task in a large sample of heterogeneous stock rats
Scientific Reports (2023)
-
The role of prefrontal cortex in cognitive control and executive function
Neuropsychopharmacology (2022)
-
A neural and behavioral trade-off between value and uncertainty underlies exploratory decisions in normative anxiety
Molecular Psychiatry (2022)
-
Cingulate cortex morphology impacts on neurofunctional activity and behavioral performance in interference tasks
Scientific Reports (2022)
-
Relationship of ventral striatum activation during effort discounting to clinical amotivation severity in schizophrenia
npj Schizophrenia (2021)