Abstract
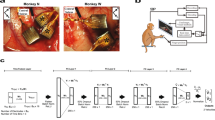
Neural prostheses translate neural activity from the brain into control signals for guiding prosthetic devices, such as computer cursors and robotic limbs, and thus offer individuals with disabilities greater interaction with the world. However, relatively low performance remains a critical barrier to successful clinical translation; current neural prostheses are considerably slower, with less accurate control, than the native arm. Here we present a new control algorithm, the recalibrated feedback intention–trained Kalman filter (ReFIT-KF) that incorporates assumptions about the nature of closed-loop neural prosthetic control. When tested in rhesus monkeys implanted with motor cortical electrode arrays, the ReFIT-KF algorithm outperformed existing neural prosthetic algorithms in all measured domains and halved target acquisition time. This control algorithm permits sustained, uninterrupted use for hours and generalizes to more challenging tasks without retraining. Using this algorithm, we demonstrate repeatable high performance for years after implantation in two monkeys, thereby increasing the clinical viability of neural prostheses.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Serruya, M.D., Hatsopoulos, N.G., Paninski, L., Fellows, M.R. & Donoghue, J.P. Instant neural control of a movement signal. Nature 416, 141–142 (2002).
Taylor, D.M., Tillery, S.I. & Schwartz, A.B. Direct cortical control of 3D neuroprosthetic devices. Science 296, 1829–1832 (2002).
Carmena, J.M. et al. Learning to control a brain-machine interface for reaching and grasping by primates. PLoS Biol. 1, E42 (2003).
Velliste, M., Perel, S., Spalding, M.C., Whitford, A.S. & Schwartz, A.B. Cortical control of a prosthetic arm for self-feeding. Nature 453, 1098–1101 (2008).
Mulliken, G.H., Musallam, S. & Andersen, R.A. Decoding trajectories from posterior parietal cortex ensembles. J. Neurosci. 28, 12913–12926 (2008).
Shpigelman, L., Lalazar, H. & Vaadia, E. Kernel-arma for hand tracking and brain-machine interfacing during 3D motor control. Adv. Neural Info. Proc. Sys. 21, 1489–1496 (2009).
Ganguly, K. & Carmena, J.M. Emergence of a stable cortical map for neuroprosthetic control. PLoS Biol. 7, e1000153 (2009).
Suminski, A.J., Tkach, D.C., Fagg, A.H. & Hatsopoulos, N.G. Incorporating feedback from multiple sensory modalities enhances brain-machine interface control. J. Neurosci. 30, 16777–16787 (2010).
Li, Z., O'Doherty, J.E., Lebedev, M.A. & Nicolelis, M.A. Adaptive decoding for brain-machine interfaces through bayesian parameter updates. Neural Comput. 23, 3162–3204 (2011).
Hochberg, L.R. et al. Neuronal ensemble control of prosthetic devices by a human with tetraplegia. Nature 442, 164–171 (2006).
Kim, S.P., Simeral, J.D., Hochberg, L.R., Donoghue, J.P. & Black, M.J. Neural control of computer cursor velocity by decoding motor cortical spiking activity in humans with tetraplegia. J. Neural Eng. 5, 455–476 (2008).
Kim, S.P. et al. Point-and-click cursor control with an intracortical neural interface system in humans with tetraplegia. IEEE Trans. Neural Syst. Rehabil. Eng. 19, 193–203 (2011).
Hochberg, L.R. et al. Reach and grasp by people with tetraplegia using a neurally controlled robotic arm. Nature 485, 372–375 (2012).
Judy, J. Neural interfaces for upper-limb prosthesis control: opportunities to improve long-term reliability. IEEE Pulse 3, 57–60 (2012).
Chestek, C.A. et al. Long-term stability of neural prosthetic control signals from silicon cortical arrays in rhesus macaque motor cortex. J. Neural Eng. 8, 045005 (2011).
Cunningham, J.P. et al. A closed-loop human simulator for investigating the role of feedback-control in brain-machine interfaces. J. Neurophysiol. 105, 1932–1949 (2011).
Churchland, M.M., Cunningham, J.P., Kaufman, M.T., Ryu, S.I. & Shenoy, K.V. Cortical preparatory activity: representation of movement or first cog in a dynamical machine? Neuron 68, 387–400 (2010).
Kaufman, M.T. et al. Roles of monkey premotor neuron classes in movement preparation and execution. J. Neurophysiol. 104, 799–810 (2010).
Sadtler, P.T., Ryu, S.I., Yu, B.M., & Batista, A.P. High-performance neural prosthetic control along instructed paths. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2011, 601–604 (2011).
Gage, G.J., Ludwig, K.A., Otto, K.J., Ionides, E.L. & Kipke, D.R. Naive coadaptive cortical control. J. Neural Eng. 2, 52–63 (2005).
Fraser, G.W., Chase, S.M., Whitford, A. & Schwartz, A.B. Control of a brain-computer interface without spike sorting. J. Neural Eng. 6, 055004 (2009).
Chase, S.M., Schwartz, A.B., & Kass, R.E. Bias, optimal linear estimation and the differences between open-loop simulation and closed-loop performance of spiking-based brain-computer interface algorithms. Neural Networks 22, 1203–1213 (2009).
Srinivasan, L., Eden, U.T., Mitter, S.K. & Brown, E.N. General-purpose filter design for neural prosthetic devices. J. Neurophysiol. 98, 2456–2475 (2007).
Yu, B.M. et al. Mixture of trajectory models for neural decoding of goal-directed movements. J. Neurophysiol. 97, 3763–3780 (2007).
Card, S.K., English, W.K. & Burr, B.J. Evaluation of mouse, rate-controlled isometric joystick, step keys, and text keys for text selection on a CRT. Ergonomics 21, 601–613 (1978).
Kalaska, J.F. From intention to action: motor cortex and the control of reaching movements. Adv. Exp. Med. Biol. 629, 139–178 (2009).
Golub, M.D., Yu, B.M. & Chase, S.M. Internal models engaged by brain-computer interface control. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2012, 1327–1330 (2012).
Chestek, C.A. et al. Neural prosthetic systems: current problems and future directions. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2009, 3369–3375 (2009).
Santhanam, G. et al. HermesB: a continuous neural recording system for freely behaving primates. IEEE Trans. Biomed. Eng. 54, 2037–2050 (2007).
Hochberg, L.R. Turning thought into action. N. Engl. J. Med. 359, 1175–1177 (2008).
McFarland, D.J., Sarnacki, W.A. & Wolpaw, J.R. Electroencephalographic (EEG) control of three-dimensional movement. J. Neural Eng. 7, 036007 (2010).
Schalk, G. et al. Two-dimensional movement control using electrocorticographic signals in humans. J. Neural Eng. 5, 75–84 (2008).
Gilja, V. et al. Challenges and opportunities for next-generation intra-cortically based neural prostheses. IEEE Trans. Biomed. Eng. 58, 1891–1899 (2011).
Santhanam, G., Ryu, S.I., Yu, B.M., Afshar, A. & Shenoy, K.V. A high-performance brain-computer interface. Nature 442, 195–198 (2006).
Acknowledgements
We thank M. Mazariegos, J. Aguayo, W. Kalkus, S. Kang, E. Morgan and C. Sherman for surgical assistance and veterinary care, D. Haven and B. Oskotsky for information technology support, S. Eisensee, B. Davis and E. Castaneda for administrative assistance, P. Ortega for mathematical insight, and S. Stavisky for data-collection assistance. This work was supported by a US National Defense Science and Engineering Graduate Fellowship (V.G.), National Science Foundation Graduate Research Fellowships (V.G., C.A.C., J.M.F., M.T.K. and J.C.K.), Stanford Medical Scholars Program, Howard Hughes Medical Institute Medical Research Fellows Program, Paul and Daisy Soros Fellowship, Stanford Medical Scientist Training Program (P.N.), Stanford Graduate Fellowship (C.A.C., J.P.C. and J.M.F.), Gatsby Charitable Foundation (B.M.Y.), a Helen Hay Whitney postdoctoral fellowship (M.M.C.), Burroughs Welcome Fund Career Awards in the Biomedical Sciences (M.M.C. and K.V.S.), the Christopher and Dana Reeve Paralysis Foundation (S.I.R. and K.V.S.), Defense Advanced Research Projects Agency Revolutionizing Prosthetics 2009 N66001-06-C-8005, Reorganization and Plasticity to Accelerate Injury Recovery N66001-10-C-2010, US National Institutes of Health, National Institute of Neurological Disorders and Stroke Collaborative Research in Computational Neuroscience grant R01-NS054283, and National Institutes of Health Directors Pioneer Award 1DP1OD006409 (K.V.S.).
Author information
Authors and Affiliations
Contributions
V.G. and P.N. were responsible for infrastructure development, animal training, and data collection and analysis. V.G. was responsible for algorithm design, supplementary modeling and writing of the paper. P.N. and B.M.Y. participated in algorithm design. C.A.C. participated in animal training, infrastructure development and data analysis. J.P.C. participated in algorithm design and infrastructure development. J.M.F. participated in data collection and analysis. M.M.C. and M.T.K. provided initial animal training for the obstacle avoidance task. J.C.K. participated in data collection. S.I.R. was responsible for surgical implantation. K.V.S. was involved in all aspects of the study.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–9, Supplementary Tables 1–2, Supplementary Modeling (PDF 3646 kb)
Supplementary Video 1
Center-out-and-back task (monkey L 1/29/2010). In this task, targets alternate between a central target and one of eight peripheral targets. Peripheral targets are evenly spaced around a ring 8 cm from the central target. Although targets and the cursor are presented as 1.4-cm-diameter spheres, the target acceptance window is a 4 cm by 4 cm square. A trial is successful and a reward is given if the cursor center is held within this acceptance window for 500 ms. The monkey has 2 s to acquire each target. This video was selected as a representative example of performance in this task. The mean time to target for the subset of trials shown in the video is 678 ms. The video was recorded during the experimental session from a monitor mirroring the monitor shown to the monkey. (MP4 2488 kb)
Supplementary Video 2
Center-out-and-back task (monkey J 3/09/2010). This video shows the same task as in Supplementary Video 1, but for the other monkey (J). Task parameters are identical except for the visually presented cursor, and the target diameter is 1.2 cm. This video was selected as a representative example of performance in this task. The mean time to target for the subset of trials shown in the video is 671 ms. The video was recorded during the experimental session from a monitor mirroring the monitor shown to the monkey. (MP4 2486 kb)
Supplementary Video 3
Pinball task (monkey L 4/12/2010). In this task, targets can appear anywhere within a 16 cm by 16 cm workspace. The center of the cursor must be held within the 4 cm by 4 cm acceptance window for 500 ms. The cursor and targets are 1.2 cm in diameter, and the monkey has 2 s to acquire each target. Data from this 100-min neural control session are presented in Figure 3. The video is a reconstruction of the graphics displayed during online control sessions, built from data logged by the behavioral control system. (MP4 101903 kb)
Supplementary Video 4
Pinball task (monkey L 4/12/2010). The video shows 20 seconds of trials taken every 10 min during the 100-min neural control session in Supplementary Video 3 (that is, 0–20 s, 10 m + 0–20 s, 20 m + 0–20 s and so on). The video is a reconstruction of the graphics displayed during online control sessions, built from data logged by the behavioral control system. (MP4 8412 kb)
Supplementary Video 5
Pinball task (monkey J 2/4/2010). This video shows the same task as Supplementary Videos 3 and 4 for the second monkey. Task parameters are identical except the monkey had 3 s to acquire each target; cursor and targets are 1.4 cm in diameter. The video is a reconstruction of the graphics displayed during online control sessions, built from data logged by the behavioral control system. (MP4 82402 kb)
Supplementary Video 6
Maze task (monkey J 3/9/2010). In this obstacle-avoidance task, a magenta barrier appears on screen and turns on at the same time as the target. The monkey must acquire and hold the target (4 cm by 4 cm acceptance window) for 500 ms without hitting the barrier. If the cursor hits the barrier, the trial is aborted. The monkey has 5 s to acquire each target. Cursor and targets are 1.2 cm in diameter. As noted in the main text, this task was challenging for the monkey in both native arm control and neural control modes, with comparable task success rates. Unlike the trials shown in the previous five videos, here the trials selected are not representative. The trials, and associated stretch of video, were selected to show a string of successful trials. The video was recorded during the experimental session from a monitor mirroring the monitor shown to the monkey. (MP4 3689 kb)
Rights and permissions
About this article
Cite this article
Gilja, V., Nuyujukian, P., Chestek, C. et al. A high-performance neural prosthesis enabled by control algorithm design. Nat Neurosci 15, 1752–1757 (2012). https://doi.org/10.1038/nn.3265
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.3265
This article is cited by
-
Brain control of bimanual movement enabled by recurrent neural networks
Scientific Reports (2024)
-
Neurotechnologies to restore hand functions
Nature Reviews Bioengineering (2023)
-
Spiking image processing unit based on neural analog of Boolean logic operations
Cognitive Neurodynamics (2023)
-
Real-time brain-machine interface in non-human primates achieves high-velocity prosthetic finger movements using a shallow feedforward neural network decoder
Nature Communications (2022)
-
Generalizable spelling using a speech neuroprosthesis in an individual with severe limb and vocal paralysis
Nature Communications (2022)