Abstract
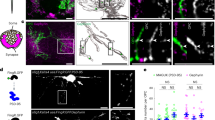
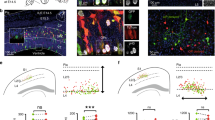
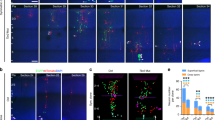
The sequential synaptic integration of adult-born neurons has been widely examined in rodents, but the mechanisms regulating the integration remain largely unknown. The primary cilium, a microtubule-based signaling center, is essential for vertebrate development, including the development of the CNS. We examined the assembly and function of the primary cilium in the synaptic integration of adult-born mouse hippocampal neurons. Primary cilia were absent in young adult-born neurons, but assembled precisely at the stage when newborn neurons approach their final destination, further extend dendrites and form synapses with entorhinal cortical projections. Conditional deletion of cilia from adult-born neurons induced severe defects in dendritic refinement and synapse formation. Deletion of primary cilia led to enhanced Wnt and β-catenin signaling, which may account for these developmental defects. Taken together, our findings identify the assembly of primary cilia as a critical regulatory event in the dendritic refinement and synaptic integration of adult-born neurons.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Altman, J. & Das, G.D. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J. Comp. Neurol. 124, 319–335 (1965).
Kempermann, G., Gast, D. & Gage, F.H. Neuroplasticity in old age: sustained fivefold induction of hippocampal neurogenesis by long-term environmental enrichment. Ann. Neurol. 52, 135–143 (2002).
Ma, D.K. et al. Epigenetic choreographers of neurogenesis in the adult mammalian brain. Nat. Neurosci. 13, 1338–1344 (2010).
Zhao, C., Deng, W. & Gage, F.H. Mechanisms and functional implications of adult neurogenesis. Cell 132, 645–660 (2008).
Ge, S., Pradhan, D.A., Ming, G.L. & Song, H. GABA sets the tempo for activity-dependent adult neurogenesis. Trends Neurosci. 30, 1–8 (2007).
Kelsch, W., Sim, S. & Lois, C. Watching synaptogenesis in the adult brain. Annu. Rev. Neurosci. 33, 131–149 (2010).
van Praag, H. et al. Functional neurogenesis in the adult hippocampus. Nature 415, 1030–1034 (2002).
Ge, S. et al. GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature 439, 589–593 (2006).
Overstreet Wadiche, L., Bromberg, D.A., Bensen, A.L. & Westbrook, G.L. GABAergic signaling to newborn neurons in dentate gyrus. J. Neurophysiol. 94, 4528–4532 (2005).
Esposito, M.S. et al. Neuronal differentiation in the adult hippocampus recapitulates embryonic development. J. Neurosci. 25, 10074–10086 (2005).
Gerdes, J.M., Davis, E.E. & Katsanis, N. The vertebrate primary cilium in development, homeostasis and disease. Cell 137, 32–45 (2009).
Lee, J.E. & Gleeson, J.G. Cilia in the nervous system: linking cilia function and neurodevelopmental disorders. Curr. Opin. Neurol. 24, 98–105 (2011).
Goetz, S.C. & Anderson, K.V. The primary cilium: a signalling centre during vertebrate development. Nat. Rev. Genet. 11, 331–344 (2010).
Lancaster, M.A. & Gleeson, J.G. The primary cilium as a cellular signaling center: lessons from disease. Curr. Opin. Genet. Dev. 19, 220–229 (2009).
Bisgrove, B.W. & Yost, H.J. The roles of cilia in developmental disorders and disease. Development 133, 4131–4143 (2006).
Han, Y.G. & Alvarez-Buylla, A. Role of primary cilia in brain development and cancer. Curr. Opin. Neurobiol. 20, 58–67 (2010).
Han, Y.G. et al. Hedgehog signaling and primary cilia are required for the formation of adult neural stem cells. Nat. Neurosci. 11, 277–284 (2008).
Breunig, J.J. et al. Primary cilia regulate hippocampal neurogenesis by mediating sonic hedgehog signaling. Proc. Natl. Acad. Sci. USA 105, 13127–13132 (2008).
Corbit, K.C. et al. Kif3a constrains beta-catenin-dependent Wnt signaling through dual ciliary and non-ciliary mechanisms. Nat. Cell Biol. 10, 70–76 (2008).
Szymczak, A.L. et al. Correction of multi-gene deficiency in vivo using a single 'self-cleaving' 2A peptide–based retroviral vector. Nat. Biotechnol. 22, 589–594 (2004).
Bishop, G.A., Berbari, N.F., Lewis, J. & Mykytyn, K. Type III adenylyl cyclase localizes to primary cilia throughout the adult mouse brain. J. Comp. Neurol. 505, 562–571 (2007).
Pazour, G.J. et al. Chlamydomonas IFT88 and its mouse homologue, polycystic kidney disease gene tg737, are required for assembly of cilia and flagella. J. Cell Biol. 151, 709–718 (2000).
Schneider, L. et al. Directional cell migration and chemotaxis in wound healing response to PDGF-AA are coordinated by the primary cilium in fibroblasts. Cell. Physiol. Biochem. 25, 279–292 (2010).
Tsai, L.H. & Gleeson, J.G. Nucleokinesis in neuronal migration. Neuron 46, 383–388 (2005).
Duan, X. et al. Disrupted-In-Schizophrenia 1 regulates integration of newly generated neurons in the adult brain. Cell 130, 1146–1158 (2007).
Amaral, D.G., Scharfman, H.E. & Lavenex, P. The dentate gyrus: fundamental neuroanatomical organization (dentate gyrus for dummies). Prog. Brain Res. 163, 3–22 (2007).
Zhang, F. et al. Multimodal fast optical interrogation of neural circuitry. Nature 446, 633–639 (2007).
Ge, S., Yang, C.H., Hsu, K.S., Ming, G.L. & Song, H. A critical period for enhanced synaptic plasticity in newly generated neurons of the adult brain. Neuron 54, 559–566 (2007).
Kempermann, G., Kuhn, H.G. & Gage, F.H. More hippocampal neurons in adult mice living in an enriched environment. Nature 386, 493–495 (1997).
Rohatgi, R., Milenkovic, L., Corcoran, R.B. & Scott, M.P. Hedgehog signal transduction by Smoothened: pharmacologic evidence for a 2-step activation process. Proc. Natl. Acad. Sci. USA 106, 3196–3201 (2009).
Willaredt, M.A. et al. A crucial role for primary cilia in cortical morphogenesis. J. Neurosci. 28, 12887–12900 (2008).
Nishimura, T. et al. Role of the PAR-3–KIF3 complex in the establishment of neuronal polarity. Nat. Cell Biol. 6, 328–334 (2004).
Brown, C.L. et al. Kinesin-2 is a motor for late endosomes and lysosomes. Traffic 6, 1114–1124 (2005).
Delaval, B., Bright, A., Lawson, N.D. & Doxsey, S. The cilia protein IFT88 is required for spindle orientation in mitosis. Nat. Cell Biol. 13, 461–468 (2011).
Robert, A. et al. The intraflagellar transport component IFT88/polaris is a centrosomal protein regulating G1-S transition in non-ciliated cells. J. Cell. Sci. 120, 628–637 (2007).
Lie, D.C. et al. Wnt signaling regulates adult hippocampal neurogenesis. Nature 437, 1370–1375 (2005).
Maretto, S. et al. Mapping Wnt/β-catenin signaling during mouse development and in colorectal tumors. Proc. Natl. Acad. Sci. USA 100, 3299–3304 (2003).
McDermott, K.M., Liu, B.Y., Tlsty, T.D. & Pazour, G.J. Primary cilia regulate branching morphogenesis during mammary gland development. Curr. Biol. 20, 731–737 (2010).
Brault, V. et al. Inactivation of the beta-catenin gene by Wnt1-Cre–mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development 128, 1253–1264 (2001).
Li, F.Q., Mofunanya, A., Fischer, V., Hall, J. & Takemaru, K. Nuclear-cytoplasmic shuttling of Chibby controls beta-catenin signaling. Mol. Biol. Cell 21, 311–322 (2010).
Budnik, V. & Salinas, P.C. Wnt signaling during synaptic development and plasticity. Curr. Opin. Neurobiol. 21, 151–159 (2011).
Chizhikov, V.V. et al. Cilia proteins control cerebellar morphogenesis by promoting expansion of the granule progenitor pool. J. Neurosci. 27, 9780–9789 (2007).
Masdeu, C., Bernard, V., Faure, H., Traiffort, E. & Ruat, M. Distribution of Smoothened at hippocampal mossy fiber synapses. Neuroreport 18, 395–399 (2007).
Yam, P.T., Langlois, S.D., Morin, S. & Charron, F. Sonic hedgehog guides axons through a noncanonical, Src family kinase–dependent signaling pathway. Neuron 62, 349–362 (2009).
Yu, X. & Malenka, R.C. Beta-catenin is critical for dendritic morphogenesis. Nat. Neurosci. 6, 1169–1177 (2003).
Gao, X., Arlotta, P., Macklis, J.D. & Chen, J. Conditional knockout of beta-catenin in postnatal-born dentate gyrus granule neurons results in dendritic malformation. J. Neurosci. 27, 14317–14325 (2007).
Rosso, S.B., Sussman, D., Wynshaw-Boris, A. & Salinas, P.C. Wnt signaling through Dishevelled, Rac and JNK regulates dendritic development. Nat. Neurosci. 8, 34–42 (2005).
Ocbina, P.J., Tuson, M. & Anderson, K.V. Primary cilia are not required for normal canonical Wnt signaling in the mouse embryo. PLoS ONE 4, e6839 (2009).
Huang, P. & Schier, A.F. Dampened Hedgehog signaling but normal Wnt signaling in zebrafish without cilia. Development 136, 3089–3098 (2009).
Spassky, N. et al. Primary cilia are required for cerebellar development and Shh-dependent expansion of progenitor pool. Dev. Biol. 317, 246–259 (2008).
Acknowledgements
We would like to thank S. Halegoua, M. Kernan, G. Matthews, L. Role and H. Song for critical comments, and F.-Q. Li, J. Rodriguez and Q. Xiong for technical support. This work was supported by grants from the US National Institutes of Health (NS065915 to S.G. and HL107493 to K.-I.T.), the American Heart Association (0930067N to S.G.) and the State University of New York Research Excellence in Academic Health (to S.G.).
Author information
Authors and Affiliations
Contributions
N.K. engineered the retroviral constructs. N.K. and J.W. carried out the immunohistochemistry and confocal imaging analysis. Y.G. performed all of the physiology analyses and took some images. S.J. helped analyze some images and edited the manuscript. K.-I.T. helped to characterize the retroviral vectors. S.G. supervised the project. S.G. and J.L. wrote the manuscript. All of the authors read and discussed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–9 (PDF 4904 kb)
Supplementary Video 1
Three dimensional reconstruction of Z-series confocal images of primary cilia. Shown is a primary cilium image of an adult-born neuron at 21 dpi expressing the dTomato and EGFP-Centrin-2, counterstained for DAPI and immunostained for ACIII (see Methods). (MOV 2018 kb)
Rights and permissions
About this article
Cite this article
Kumamoto, N., Gu, Y., Wang, J. et al. A role for primary cilia in glutamatergic synaptic integration of adult-born neurons. Nat Neurosci 15, 399–405 (2012). https://doi.org/10.1038/nn.3042
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.3042
This article is cited by
-
Supporting Evidence of Human Enteric Nervous System Adult Neurogenesis: Presence of Primary Cilia and Adult Neurogenesis Markers
Cellular and Molecular Neurobiology (2022)
-
Distinct regulation of hippocampal neuroplasticity and ciliary genes by corticosteroid receptors
Nature Communications (2021)
-
Primary cilia mediate early life programming of adiposity through lysosomal regulation in the developing mouse hypothalamus
Nature Communications (2020)
-
Dentate gyrus circuits for encoding, retrieval and discrimination of episodic memories
Nature Reviews Neuroscience (2020)
-
Effects of synaptic integration on the dynamics and computational performance of spiking neural network
Cognitive Neurodynamics (2020)