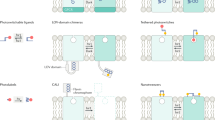
Abstract
Light-activated ion channels provide a precise and noninvasive optical means for controlling action potential firing, but the genes encoding these channels must first be delivered and expressed in target cells. Here we describe a method for bestowing light sensitivity onto endogenous ion channels that does not rely on exogenous gene expression. The method uses a synthetic photoisomerizable small molecule, or photoswitchable affinity label (PAL), that specifically targets K+ channels. PALs contain a reactive electrophile, enabling covalent attachment of the photoswitch to naturally occurring nucleophiles in K+ channels. Ion flow through PAL-modified channels is turned on or off by photoisomerizing PAL with different wavelengths of light. We showed that PAL treatment confers light sensitivity onto endogenous K+ channels in isolated rat neurons and in intact neural structures from rat and leech, allowing rapid optical regulation of excitability without genetic modification.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Callaway, E.M. & Yuste, R. Stimulating neurons with light. Curr. Opin. Neurobiol. 12, 587–592 (2002).
Kramer, R.H., Chambers, J.J. & Trauner, D. Photochemical tools for remote control of ion channels in excitable cells. Nat. Chem. Biol. 1, 360–365 (2005).
Miesenbock, G. & Kevrekidis, I.G. Optical imaging and control of genetically designated neurons in functioning circuits. Annu. Rev. Neurosci. 28, 533–563 (2005).
Zhang, F., Wang, L.P., Boyden, E.S. & Deisseroth, K. Channelrhodopsin-2 and optical control of excitable cells. Nat. Methods 3, 785–792 (2006).
Ellis-Davies, G.C. Caged compounds: photorelease technology for control of cellular chemistry and physiology. Nat. Methods 4, 619–628 (2007).
Dalva, M.B. & Katz, L.C. Rearrangements of synaptic connections in visual cortex revealed by laser photostimulation. Science 265, 255–258 (1994).
Callaway, E.M. & Katz, L.C. Photostimulation using caged glutamate reveals functional circuitry in living brain slices. Proc. Natl. Acad. Sci. USA 90, 7661–7665 (1993).
Shepherd, G.M., Pologruto, T.A. & Svoboda, K. Circuit analysis of experience-dependent plasticity in the developing rat barrel cortex. Neuron 38, 277–289 (2003).
Nikolenko, V., Poskanzer, K.E. & Yuste, R. Two-photon photostimulation and imaging of neural circuits. Nat. Methods 4, 943–950 (2007).
Banghart, M., Borges, K., Isacoff, E., Trauner, D. & Kramer, R.H. Light-activated ion channels for remote control of neuronal firing. Nat. Neurosci. 7, 1381–1386 (2004).
Volgraf, M. et al. Allosteric control of an ionotropic glutamate receptor with an optical switch. Nat. Chem. Biol. 2, 47–52 (2006).
Szobota, S. et al. Remote control of neuronal activity with a light-gated glutamate receptor. Neuron 54, 535–545 (2007).
Zhang, F. et al. Multimodal fast optical interrogation of neural circuitry. Nature 446, 633–639 (2007).
Boyden, E.S., Zhang, F., Bamberg, E., Nagel, G. & Deisseroth, K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci. 8, 1263–1268 (2005).
Bi, A. et al. Ectopic expression of a microbial-type rhodopsin restores visual responses in mice with photoreceptor degeneration. Neuron 50, 23–33 (2006).
Li, X. et al. Fast noninvasive activation and inhibition of neural and network activity by vertebrate rhodopsin and green algae channelrhodopsin. Proc. Natl. Acad. Sci. USA 102, 17816–17821 (2005).
Han, X. & Boyden, E.S. Multiple-color optical activation, silencing, and desynchronization of neural activity, with single-spike temporal resolution. PLoS ONE 2, e299 (2007).
Petreanu, L., Huber, D., Sobczyk, A. & Svoboda, K. Channelrhodopsin-2-assisted circuit mapping of long-range callosal projections. Nat. Neurosci. 10, 663–668 (2007).
Arenkiel, B.R. et al. In vivo light-induced activation of neural circuitry in transgenic mice expressing channelrhodopsin-2. Neuron 54, 205–218 (2007).
Nagel, G. et al. Light activation of channelrhodopsin-2 in excitable cells of Caenorhabditis elegans triggers rapid behavioral responses. Curr. Biol. 15, 2279–2284 (2005).
Wang, H. et al. High-speed mapping of synaptic connectivity using photostimulation in channelrhodopsin-2 transgenic mice. Proc. Natl. Acad. Sci. USA 104, 8143–8148 (2007).
Schroll, C. et al. Light-induced activation of distinct modulatory neurons triggers appetitive or aversive learning in Drosophila larvae. Curr. Biol. 16, 1741–1747 (2006).
Wold, F. Affinity labeling–an overview. Methods Enzymol. 46, 3–14 (1977).
Gorostiza, P. et al. Mechanisms of photoswitch conjugation and light activation of an ionotropic glutamate receptor. Proc. Natl. Acad. Sci. USA 104, 10865–10870 (2007).
Nadim, F. & Calabrese, R.L. A slow outward current activated by FMRFamide in heart interneurons of the medicinal leech. J. Neurosci. 17, 4461–4472 (1997).
Hill, A.A., Lu, J., Masino, M.A., Olsen, O.H. & Calabrese, R.L. A model of a segmental oscillator in the leech heartbeat neuronal network. J. Comput. Neurosci. 10, 281–302 (2001).
Hattar, S., Liao, H.W., Takao, M., Berson, D.M. & Yau, K.W. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science 295, 1065–1070 (2002).
Lakhanpal, R.R. et al. Advances in the development of visual prostheses. Curr. Opin. Ophthalmol. 14, 122–127 (2003).
Xia, Z., Dudek, H., Miranti, C.K. & Greenberg, M.E. Calcium influx via the NMDA receptor induces immediate early gene transcription by a MAP kinase/ERK-dependent mechanism. J. Neurosci. 16, 5425–5436 (1996).
Hoshi, T., Zagotta, W.N. & Aldrich, R.W. Biophysical and molecular mechanisms of Shaker potassium channel inactivation. Science 250, 533–538 (1990).
An, W.F. et al. Modulation of A-type potassium channels by a family of calcium sensors. Nature 403, 553–556 (2000).
Higgins, D. & Banker, G.A. Primary dissociated cell cultures. in Culturing Nerve Cells (eds. Banker, G.A. & Goslin, K.) 37–78 (MIT Press, Cambridge, Massachusetts, 1998).
Masino, M.A. & Calabrese, R.L. Phase relationships between segmentally organized oscillators in the leech heartbeat pattern generating network. J. Neurophysiol. 87, 1572–1585 (2002).
Acknowledgements
We thank E. Isacoff, J. Chambers, S.-Y. Choi and S. Jackman for helpful comments, J. Flannery and K. Greenberg for help with RGC experiments, D. Johnston (University of Texas at Austin), B. Rothberg (University of Texas Health Science Center at San Antonio), B. Rudy (New York University), W. Catterall (University of Washington) and J. Trimmer (University of California Davis) for providing plasmids. This work was supported by the Howard Hughes Medical Institute (K.B.), the US National Science Foundation (IOB-0523959 to W.B.K.), Microsoft Research Labs (to W.B.K.) and the US National Institutes of Health (GM057027 to D.T., MH43396 to W.B.K., and EY16249 to R.H.K.). D.A.W., Q.G. and W.B.K. thank A. Blankenship and M. Feller for the loan of their xenon lamp.
Author information
Authors and Affiliations
Corresponding authors
Supplementary information
Supplementary Text and Figures
Supplementary Figure 1, Supplementary Methods (PDF 468 kb)
Rights and permissions
About this article
Cite this article
Fortin, D., Banghart, M., Dunn, T. et al. Photochemical control of endogenous ion channels and cellular excitability. Nat Methods 5, 331–338 (2008). https://doi.org/10.1038/nmeth.1187
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmeth.1187
This article is cited by
-
New tricks and emerging applications from contemporary azobenzene research
Photochemical & Photobiological Sciences (2022)
-
Advances and opportunities in the exciting world of azobenzenes
Nature Reviews Chemistry (2021)
-
Neuronal firing modulation by a membrane-targeted photoswitch
Nature Nanotechnology (2020)
-
Optical control of neuronal ion channels and receptors
Nature Reviews Neuroscience (2019)
-
Photopharmacology: A Brief Review Using the Control of Potassium Channels as an Example
Neuroscience and Behavioral Physiology (2019)