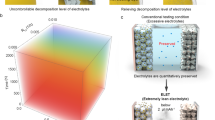
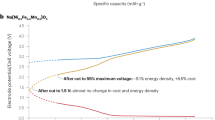
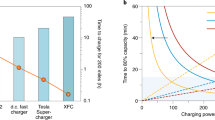
Abstract
The development of improved rechargeable batteries represents a major technological challenge for this new century, as batteries constitute the limiting components in the shift from petrol (gasoline) powered to electric vehicles, while also enabling the use of more renewable energy on the grid. To minimize the ecological implications associated with their wider use, we must integrate sustainability of battery materials into our research endeavours, choosing chemistries that have a minimum footprint in nature and that are more readily recycled or integrated into a full circular economy. Sustainability and cost concerns require that we greatly increase the battery lifetime and consider second lives for batteries. As part of this, we must monitor the state of health of batteries continuously during operation to minimize their degradation. It is thus important to push the frontiers of operando techniques to monitor increasingly complex processes. In this Review, we will describe key advances in both more sustainable chemistries and operando techniques, along with some of the remaining challenges and possible solutions, as we personally perceive them.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Larcher, D. & Tarascon, J. M. Towards greener and more sustainable batteries for electrical energy storage. Nat. Chem. 7, 19–29 (2015).
US Energy Information Administration. Annual Energy Outlook (2016); http://go.nature.com/2gh0oZl
Pillot, C. Avicenne Energy Analysis (April 2016).
Ishihara, K., Kihira, N., Terada, N., Iwahori, T. & Nishimura, K. Life cycle analysis of large-size lithium-ion secondary batteries developed in the Japanese national project. In Proceedings from 5th Ecobalance Conference, 293–294 (2002).
Straubel, J. B. Energy storage, EV's and the grid. EIA Conf., Washington DC, 15 June 2015; http://go.nature.com/2gDNTcR
Nykvist, B. & Nilsson, M. Rapidly falling costs of battery packs for electric vehicles. Nat. Clim. Change 5, 329–332 (2015).
Viswanathan, V. V. & Kintner-Meyer, M. Second use of transportation batteries: maximizing the value of batteries for transportation and grid services. IEEE Trans. Vehic. Technol. 60, 2963–2970 (2011).
Jiao, N. Evans, S., Secondary use of electric vehicle batteries and potential impacts on business models. J. Indust. Prod. Eng. 33, 348–354 (2016).
Zhang, P., Yokoyama, T., Itabashi, O., Suzuki, T. M. & Inoue, K. Hydrometallurgical process for recovery of metal values from spent lithium-ion secondary batteries. Hydrometallurgy 47, 259–271 (1998).
Padhi, A. K., Nanjundaswamy, K. S., Masquelier, C., Okada, S. & Goodenough, J. B. Effect of structure on the Fe3+/Fe2+ redox couple in iron phosphates. J. Electrochem. Soc. 144, 1609–1613 (1997).
Tarascon, J. M. et al. Hunting for better Li-based electrode materials via low temperature inorganic synthesis. Chem. Mater. 22, 724–739 (2009).
Ravet, N. et al. Improved iron based cathode material. In Proc. 196th ECS Meeting, Honolulu, October 1999. Extended abstract 127 (1999).
Yabuuchi, N. & Ohzuku, T. Novel lithium insertion material of LiCo1/3Ni1/3Mn1/3O2 for advanced lithium-ion batteries. J. Power Sources 119, 171–174 (2003).
Nytén, A., Abouimrane, A., Armand, M., Gustafsson, T. & Thomas, J. O. Electrochemical performance of Li2FeSiO4 as a new Li-battery cathode material. Electrochem. Commun. 7, 156–160 (2005).
Legagneur, V. et al. LiMBO3 (M = Mn, Fe, Co): synthesis, crystal structure and lithium deinsertion/insertion properties. Solid State Ion. 139, 37–46 (2001).
Rousse, G. & Tarascon, J. M. Sulfate-based polyanionic compounds for Li-ion batteries: synthesis, crystal chemistry, and electrochemistry aspects. Chem. Mater. 26, 394–406 (2014).
Masquelier, C. & Croguennec, L. Polyanionic (phosphates, silicates, sulfates) frameworks as electrode materials for rechargeable Li (or Na) batteries. Chem. Rev. 113, 6552–6591 (2013).
Ravnsbæk, D. B. et al. Extended solid solutions and coherent transformations in nanoscale olivine cathodes. Nano Lett. 14, 1484–1491 (2014).
Dewulf, J. et al. Recycling rechargeable lithium ion batteries: critical analysis of natural resource savings. Resour. Conserv. Recycl. 54, 229–234 (2010).
Ueda, A. & Ohzuku, T. Solid-state redox reactions of LiNi1/2Co1/2O2 (R3–m) for 4 volt secondary lithium cells. J. Electrochem. Soc. 141, 2010–2014 (1994).
Sun, Y.-K. et al. High-energy cathode material for long-life and safe lithium batteries. Nat. Mater. 8, 320–324 (2009).
Sun, Y.-K. et al. Nanostructured high-energy cathode materials for advanced lithium batteries. Nat. Mater. 11, 942–947 (2012).
Thackeray, M. M., Johnson, C. S., Vaughey, J. T., Li, N. & Hackney, S. A. Advances in manganese-oxide 'composite' electrodes for lithium-ion batteries. J. Mater. Chem. 15, 2257–2267 (2005).
Lu, Z., Beaulieu, L. Y., Donaberger, R. A., Thomas, C. L. & Dahn, J. R. Synthesis, structure, and electrochemical behavior of Li[NixLi1/3−2x/3Mn2/3−x/3]O2 . J. Electrochem. Soc. 149, A778–A791 (2002).
Sathiya, M. et al. Reversible anionic redox chemistry in high-capacity layered-oxide electrodes. Nat. Mater. 12, 827–835 (2013).
McCalla, E. et al. Visualization of O–O peroxo-like dimers in high-capacity layered oxides for Li-ion batteries. Science 350, 1516–1521 (2015).
Luo, K. et al. Charge-compensation in 3d-transition-metal-oxide intercalation cathodes through the generation of localized electron holes on oxygen. Nat. Chem. 8, 684 (2016).
Freire, M. et al. A new active Li–Mn–O compound for high energy density Li-ion batteries. Nat. Mater. 15, 173–177 (2016).
Obrovac, M. N. & Chevrier, V. L. Alloy negative electrodes for Li-ion batteries. Chem. Rev. 114, 11444–11502 (2014).
Buqa, H., Holzapfel, M., Krumeich, F., Veit, C. & Novák, P. Study of styrene butadiene rubber and sodium methyl cellulose as binder for negative electrodes in lithium-ion batteries. J. Power Sources 161, 617–622 (2006).
Hochgatterer, N. S. et al. Silicon/graphite composite electrodes for high-capacity anodes: influence of binder chemistry on cycling stability. Electrochem. Solid-State Lett. 11, A76–A80 (2008).
Kovalenko, I. et al. A major constituent of brown algae for use in high-capacity Li-ion batteries. Science 334, 75–79 (2011).
Chen, H. et al. From biomass to a renewable LixC6O6 organic electrode for sustainable Li-ion batteries. ChemSusChem 1, 348–355 (2008).
Poizot, P. & Dolhem, F. Clean energy new deal for a sustainable world: from non-CO2 generating energy sources to greener electrochemical storage devices. Energy Environ. Sci. 4, 2003–2019 (2011).
Yabuuchi, N., Kubota, K., Dahbi, M. & Komaba, S. Research development on sodium-ion batteries. Chem. Rev. 114, 11636–11682 (2014).
Kundu, D., Talaie, E., Duffort, V. & Nazar, L. F. The emerging chemistry of sodium ion batteries for electrochemical energy storage. Angew. Chem. Int. Ed., 54, 3431–3448 (2015).
Yabuuchi, N. et al. P2-type Nax[Fe1/2Mn1/2]O2 made from earth-abundant elements for rechargeable Na batteries. Nat. Mater. 11, 512–517 (2012).
Bianchini, M. et al. Na3V2(PO4)2F3 revisited: a high resolution diffraction study. Chem Mater. 29, 4238–4247 (2014).
Dugas, R., Zhang, B., Rozier, P. & Tarascon, J. M. Optimization of Na-ion battery systems based on polyanionic or layered positive electrodes and carbon anodes. J. Electrochem. Soc. 163, A867–A874 (2016).
Ponrouch, A. et al. Towards high energy density sodium ion batteries through electrolyte optimization. Energy Environ. Sci. 6, 2361–2369 (2013).
Le Conseil d'Administration d'Eco-Emballages nomme Philippe-Loïc Jacob au poste de Président-Directeur Général de la société. Eco-Emballages (26 November 2015); http://go.nature.com/2gkPwvc
Barker, J. et al. Low Cost Na-ion Battery Technology (Faradion, 2014); http://go.nature.com/2fuxOV8
Mohtadi, R. & Mizuno, F. Magnesium batteries: current state of the art, issues and future perspectives. Beilstein J. Nanotechnol. 5, 1291–1311 (2014).
Muldoon, J., Bucur, C. B. & Gregory, T. Quest for nonaqueous multivalent secondary batteries: magnesium and beyond. Chem. Rev. 114, 11683–11720 (2014).
Sun, X. et al. A high capacity thiospinel cathode for Mg batteries. Energy Environ. Sci. 9, 2273–2277 (2016).
Sun, X., Duffort, V., Mehdi, B. L., Browning, N. D. & Nazar, L. F. Investigation of the mechanism of Mg insertion in birnessite in non-aqueous and aqueous rechargeable Mg-ion batteries. Chem. Mater. 28, 534–542 (2016).
Yagi, S. et al. A concept of dual-salt polyvalent-metal storage battery. J. Mater. Chem. A 2, 1144–1149 (2014).
Murgia, F., Stievano, L., Monconduit, L. & Berthelot, R. Insight into the electrochemical behavior of micrometric Bi and Mg3Bi2 as high performance negative electrodes for Mg batteries. J. Mater. Chem. A, 3, 16478–16485 (2015).
Tutusaus, O. & Mohtadi, R. Paving the way towards highly stable and practical electrolytes for rechargeable magnesium batteries. ChemElectroChem 2, 51–57 (2015).
Tutusaus, O. et al. An efficient halogen-free electrolyte for use in rechargeable magnesium batteries. Angew. Chem. Int. Ed., 54, 7900–7904 (2015).
Aurbach, D. et al. Prototype systems for rechargeable magnesium batteries. Nature 407, 724–727 (2000).
Ponrouch, A., Frontera, C., Bardé, F. & Palacín, M. R. Towards a calcium-based rechargeable battery. Nat. Mater. 15, 169–172 (2016).
Abraham, K. M. & Jiang, Z. A polymer electrolyte-based rechargeable lithium/oxygen battery. J. Electrochem. Soc. 143, 1–5 (1996).
Ogasawara, T., Débart, A., Holzapfel, M., Novák, P. & Bruce, P. G. Rechargeable Li2O2 electrode for lithium batteries. J. Am. Chem. Soc. 128, 1390–1393 (2006).
Bruce, P. G., Freunberger, S. A., Hardwick, L. J. & Tarascon, J.-M. High energy storage Li–O2 and Li–S batteries. Nat. Mater. 11, 19–29 (2011).
Adams, B.D. et al., Current density dependence of peroxide formation in the Li–O2 battery and its effect on charge. Energy Environ. Sci. 6, 1772 (2013).
Johnson, L. et al. The role of LiO2 solubility in O2 reduction in aprotic solvents and its consequences for Li–O2 batteries. Nat. Chem. 6, 1091–1099 (2014).
Aetukuri, N. B. et al. Solvating additives drive solution-mediated electrochemistry and enhance toroid growth in non-aqueous Li–O2 batteries. Nat. Chem. 7, 50–56 (2015).
Chen, Y., Freunberger, S. A, Peng, Z., Fontaine, O. & Bruce, P. G. Charging a Li-O2 battery using a redox mediator. Nat. Chem. 5, 489–494 (2013).
Bergner, B. J., Schu, A., Peppler, K., Garsuch, A. & Janek, J. TEMPO: a mobile catalyst for rechargeable Li-O2 batteries. J. Am. Chem. Soc. 136, 15054–15064 (2014).
Lim, H.-D. et al. Superior rechargeability and efficiency of lithium–oxygen batteries: hierarchical air electrode architecture combined with a soluble catalyst. Angew. Chem. Int. Ed. 53, 3926–3931 (2014).
Zhangquan, P., Freunberger, S. A., Chen, Y. & Bruce, P. G. A reversible and higher-rate Li-O2 battery, Science 337, 563–566 (2012).
Thotiyl, M. M. et al. A stable cathode for the aprotic Li–O2 battery. Nat. Mater. 12, 1050–1056 (2013).
Walker, W. et al. A rechargeable Li−O2 battery using a lithium nitrate/N,N-dimethylacetamide electrolyte. J. Am. Chem. Soc. 135, 2076–2079 (2013).
Giordani, V. et al. A molten salt lithium–oxygen battery. J. Am. Chem. Soc. 138, 2658–2663 (2016).
Liu, T. et al. Cycling Li–O2 batteries via LiOH formation and decomposition. Science 350, 530–533 (2015).
Lu, J. et al. A lithium–oxygen battery based on lithium superoxide. Nature 529, 377–382 (2016).
Lim, H.-K. et al. Toward a lithium−“air” battery: the effect of CO2 on the chemistry of a lithium−oxygen cell. J. Am. Chem. Soc. 135, 9733–9742 (2013).
Hartmann P. et al. A rechargeable room-temperature sodium superoxide (NaO2) battery. Nat. Mater. 12, 228–232 (2013).
McCloskey, B. D., Garcia, J. M. & Luntz, A. C. Chemical and electrochemical differences in nonaqueous Li−O2 and Na−O2 batteries. J. Phys. Chem. Lett. 5, 1230–1235 (2014).
Fang, X. & Peng, H. A revolution in electrodes: recent progress in rechargeable lithium–sulfur batteries. Small 11, 1488–1511 (2015).
Borchardt, L., Oschatz, M. & Kaskel, S. Carbon materials for lithium sulfur batteries — critical questions, Chem. Eur. J. 22, 1–29 (2016).
Ji, X., Evers, S., Black, R. & Nazar, L. F. Stabilizing lithium–sulphur cathodes using polysulphide reservoirs. Nat. Commun. 2, 325 (2011).
Demir-Cakan, R. et al. Cathode composites for Li–S batteries via the use of oxygenated porous architectures. J. Am. Chem. Soc. 133, 16154–16160 (2011).
Pang, Q., Kundu, D., Cuisinier, M. & Nazar, L. F. Surface-enhanced redox chemistry of polysulphides on a metallic and polar host for lithium–sulphur batteries. Nat. Commun. 5, 4759 (2014).
Fan, Q., Liu, W., Weng, Z., Sun, Y. & Wang, H. Ternary hybrid material for high-performance lithium–sulfur battery. J. Am. Chem. Soc. 137, 12946–12953 (2015).
Li, W., Dahn, J. R. & Wainwright D. S. Rechargeable lithium batteries with aqueous electrolytes. Science 264, 1115–1118 (1994).
Wu, W., Mohamed, A. & Whitacre, J. F. Microwave synthesized NaTi2(PO4)3 as an aqueous sodium-ion negative electrode. J. Electrochem. Soc. 160, A497–A504 (2013).
Visco, S. J., Katz, B. D., Nimon, Y. S. & De Jonghe, L. C. Li/air non-aqueous batteries. US patent 20070117007 (2007).
Visco, S. J. et al. Aqueous electrolyte lithium sulfur batteries. US patent 20130122334 (2013).
Toussaint, G., Stevens, P., Akrour, L., Rouget R. & Fourgeot, F. Development of a rechargeable zinc–air battery. ECS Trans. 28, 25–34 (2010).
Pan, H. et al. Reversible aqueous zinc/manganese oxide energy storage from conversion reactions. Nat. Energy 1, 16039 (2016).
Ponce de Leόn, C., Frías-Ferrerb, A., González-Garcíab, J., Szántoc, D. A. & Walsh, F. C. Redox flow cells for energy conversion. J. Power Sources 160, 716–732 (2006).
Darling, R. M., Gallagher, K. G., Kowalski, J. A., Ha, S. & Brushett, F. R. Pathways to low-cost electrochemical energy storage: a comparison of aqueous and nonaqueous flow batteries. Energy Environ. Sci. 7, 3459–3477 (2014).
Chiang, Y. M., Cater, W. C., Ho, B. H. & Duduta, M. High energy density redox flow device. US patent 20100047671 A1 (2010).
Lu, Y., Goodenough, J. B. & Kim, Y. Aqueous cathode for next-generation alkali-ion batteries. J. Am. Chem. Soc. 133, 5756–5759 (2011).
Brushett, F. R., Vaughey, J. T. & Jansen, A. N. An all-organic non-aqueous lithium-ion redox flow battery. Adv. Energ. Mater. 2, 1390–1396 (2012).
Suo, L. et al. 'Water-in-salt' electrolyte enables high-voltage aqueous lithium-ion chemistries. Science 350, 938–943 (2015).
Yu, Y. et al. Dependence on crystal size of the nanoscale chemical phase distribution and fracture in LixFePO4 . Nano Lett. 15, 4282–4288 (2015).
Liu, H. et al. Capturing metastable structures during high rate cycling of LiFePO4 nanoparticle electrodes. Science 344, 1–7 (2014).
Strobridge, F. C. et al. Mapping the inhomogeneous electrochemical reaction through porous LiFePO4-electrodes in a standard coin cell battery. Chem. Mater. 27, 2374–2386 (2015).
Senyshyn, A., Mühlbauer, M. J., Nikolowski, K., Pirling, T. & Ehrenberg H. 'In-operando' neutron scattering studies on Li-ion batteries. J. Power Sources 203, 126–129 (2012).
Ogata, K. et al. Revealing lithium–silicide phase transformations in nano-structured silicon-based lithium ion batteries via in situ NMR spectroscopy. Nat. Commun. 5, 3217 (2014).
Sathiya, M. et al. Electron paramagnetic resonance imaging for real-time monitoring of Li-ion batteries. Nat. Commun. 6, 6276 (2015).
Chandrashekar, S. et al. 7Li MRI of Li batteries reveals location of microstructural lithium. Nat. Mater. 11, 311–315 (2012).
Wang, F. et al. Tracking lithium transport and electrochemical reactions in nanoparticles. Nat. Commun. 3, 1201 (2012).
Zhang, X. et al. Rate-induced solubility and suppression of the first-order transition in olivine LiFePO4 . Nano Lett. 14, 2279–2285 (2014).
Key, B. et al. Real-time NMR investigations of structural changes in silicon electrodes for lithium-ion batteries, J. Am. Chem. Soc. 131, 9239–9249, (2009).
Blanc, F., Leskes, M. & C. P. Grey, C. P. In situ solid-state NMR spectroscopy of electrochemical cells: batteries, supercapacitors, and fuel cells. Acc. Chem. Res. 46, 1952–1963, (2013).
Chang, H. J. et al. Correlating microstructural lithium metal growth with electrolyte salt depletion in lithium batteries using Li-7 MRI. J. Am. Chem. Soc. 137, 15209–15216 (2015).
Huang, J. Y. et al. In situ observation of the electrochemical lithiation of a single SnO2 nanowire electrode. Science 330, 1515–1520 (2010).
Chao, S.-C. et al. A study on the interior microstructures of working Sn particle electrode of Li-ion batteries by in situ X-ray transmission microscopy. Electrochem. Commun. 12, 234–237 (2010).
Ulvestad, A. et al. Topological defect dynamics in operando battery nanoparticles, Science 348, 6241 (2015).
Zhou, L., Leskes, M., Liu, T. & Grey, C. P. Probing dynamic processes in lithium-ion batteries by in situ NMR spectroscopy: application to Li1.08Mn1.92O4 electrodes. Angew. Chem. Int. Ed. Engl. 54, 14782–15786 (2015).
Balke, N. et al. Nanoscale mapping of ion diffusion in a lithium-ion battery cathode. Nat. Nanotechnol. 5 749–54 (2010).
Day, R. P. et al. Differential thermal analysis of Li-ion cells as an effective probe of liquid electrolyte evolution during aging. J. Electrochem. Soc. 162, A2577–A2581 (2015).
Rossini, A. J. et al. Dynamic nuclear polarization surface enhanced NMR spectroscopy. Acc. Chem. Res. 46, 1942–1951 (2013).
McBreen, J. The application of synchrotron techniques to the study of lithium-ion batteries. J. Solid State Electrochem. 13, 1051–1061 (2009).
Verma, P., Maire, P. & Novak, P. A review of the features and analyses of the solid electrolyte interphase in Li-ion batteries. Electrochem. Acta 55, 6332–6341 (2010).
Cabo-Fernandez, L., Mueller, F., Passerini, S. & Hardwick, L. J. In situ Raman spectroscopy of carbon-coated ZnFe2O4 anode material in Li-ion batteries — investigation of SEI growth. Chem. Commun. 52, 3970 (2016).
Sommer, L. W. et al. Monitoring of intercalation stages in lithium-ion cells over charge-discharge cycles with fiber optic sensors. J. Electrochem. Soc. 162, A2664–A2669 (2015).
LeVine, S. You could be driving an electric car a lot sooner than you think. Quartz (21 October 2015); http://go.nature.com/2eJT7Cc
Acknowledgements
J.-M.T. acknowledges funding from the European Research Council (ERC) (FP/2014-2020)/ERC Grant-Project670116-ARPEMA. He thanks L. Zhang and D.A. Dalla Corte for help with drawing some of the figures and members of the RS2E for discussions. C.P.G. thanks the SUPERGEN Energy Storage Hub (EP/L019469/1) and the Northeastern Center for Chemical Energy Storage, an Energy Frontier Research Center funded by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences under Award Number DE-SC0001294 for support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Grey, C., Tarascon, J. Sustainability and in situ monitoring in battery development. Nature Mater 16, 45–56 (2017). https://doi.org/10.1038/nmat4777
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat4777
This article is cited by
-
Operando monitoring of dendrite formation in lithium metal batteries via ultrasensitive tilted fiber Bragg grating sensors
Light: Science & Applications (2024)
-
Lithium-induced graphene layer containing Li3P alloy phase to achieve ultra-stable electrode interface for lithium metal anode
Rare Metals (2024)
-
Trend of Developing Aqueous Liquid and Gel Electrolytes for Sustainable, Safe, and High-Performance Li-Ion Batteries
Nano-Micro Letters (2024)
-
A Molecular-Sieving Interphase Towards Low-Concentrated Aqueous Sodium-Ion Batteries
Nano-Micro Letters (2024)
-
Mn-based cathode materials for rechargeable batteries
Science China Chemistry (2024)