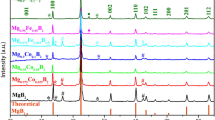
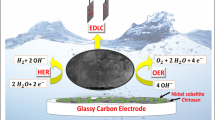
Abstract
The future of energy supply depends on innovative breakthroughs regarding the design of cheap, sustainable and efficient systems for the conversion and storage of renewable energy sources. The production of hydrogen through water splitting seems a promising and appealing solution. We found that a robust nanoparticulate electrocatalytic material, H2–CoCat, can be electrochemically prepared from cobalt salts in a phosphate buffer. This material consists of metallic cobalt coated with a cobalt-oxo/hydroxo-phosphate layer in contact with the electrolyte and mediates H2 evolution from neutral aqueous buffer at modest overpotentials. Remarkably, it can be converted on anodic equilibration into the previously described amorphous cobalt oxide film (O2–CoCat or CoPi) catalysing O2 evolution. The switch between the two catalytic forms is fully reversible and corresponds to a local interconversion between two morphologies and compositions at the surface of the electrode. After deposition, the noble-metal-free coating thus functions as a robust, bifunctional and switchable catalyst.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Lewis, N. S. & Nocera, D. G. Powering the planet: Chemical challenges in solar energy utilization. Proc. Natl Acad. Sci. USA 103, 15729–15735 (2007); 104, 20142 (2007).
Armaroli, N. & Balzani, V. The hydrogen issue. ChemSusChem 4, 21–36 (2011).
Gordon, R. B., Bertram, M. & Graedel, T. E. Metal stocks and sustainability. Proc. Natl Acad. Sci. USA 103, 1209–1214 (2006).
Kanan, M. W. & Nocera, D. G. In situ formation of an oxygen-evolving catalyst in neutral water containing phosphate and Co2+. Science 321, 1072–1075 (2008).
Yin, Q. S. et al. A fast soluble carbon-free molecular water oxidation catalyst based on abundant metals. Science 328, 342–345 (2010).
Risch, M. et al. Cobalt-oxo core of a water-oxidizing catalyst film. J. Am. Chem. Soc. 131, 6936–6937 (2009).
Dau, H. et al. The mechanism of water oxidation: From electrolysis via homogeneous to biological catalysis. ChemCatChem 2, 724–761 (2010).
Jiao, F. & Frei, H. Nanostructured cobalt and manganese oxide clusters as efficient water oxidation catalysts. Energy Environ. Sci. 3, 1018–1027 (2010).
Shevchenko, D., Anderlund, M. F., Thapper, A. & Styring, S. Photochemical water oxidation with visible light using a cobalt containing catalyst. Energy Environ. Sci. 4, 1284–1287 (2011).
Wee, T-L. et al. Photochemical synthesis of a water oxidation catalyst based on cobalt nanostructures. J. Am. Chem. Soc. 133, 16742–16745 (2011).
Chou, N. H., Ross, P. N., Bell, A. T. & Don Tilley, T. Comparison of cobalt-based nanoparticles as electrocatalysts for water oxidation. ChemSusChem 11, 1566–1569 (2011).
Dinca, M., Surendranath, Y. & Nocera, D. G. Nickel-borate oxygen-evolving catalyst that functions under benign conditions. Proc. Natl Acad. Sci. USA 107, 10337–10341 (2010).
Zaharieva, I. et al. Synthetic manganese-calcium oxides mimic the water-oxidizing complex of photosynthesis functionally and structurally. Energy Environ. Sci. 4, 2400–2408 (2011).
Tran, P. D. et al. Noncovalent modification of carbon nanotubes with pyrene-functionalized nickel complexes: Carbon monoxide tolerant catalysts for hydrogen evolution and uptake. Angew. Chem. Int. Ed. 50, 1371–1374 (2011).
Le Goff, A. et al. From hydrogenases to noble metal-free catalytic nanomaterials for H2 production and uptake. Science 326, 1384–1387 (2009).
Merki, D. & Hu, X. Recent developments of molybdenum and tungsten sulfides as hydrogen evolution catalysts. Energy Environ. Sci. 4, 3878–3888 (2011).
Hou, Y. D. et al. Bioinspired molecular co-catalysts bonded to a silicon photocathode for solar hydrogen evolution. Nature Mater. 10, 434–438 (2011).
Artero, V., Chavarot-Kerlidou, M. & Fontecave, M. Splitting water with cobalt. Angew. Chem. Int. Ed. 50, 7238–7266 (2011).
Baffert, C., Artero, V. & Fontecave, M. Cobaloximes as functional models for hydrogenases. 2. proton electroreduction catalyzed by difluoroborylbis(dimethylglyoximato)cobalt(II) complexes in organic media. Inorg. Chem. 46, 1817–1824 (2007).
Jacques, P-A., Artero, V., Pécaut, J. & Fontecave, M. Cobalt and nickel diimine-dioxime complexes as molecular electrocatalysts for hydrogen evolution with low overvoltages. Proc. Natl Acad. Sci. USA 106, 20627–20632 (2009).
Razavet, M., Artero, V. & Fontecave, M. Proton electroreduction catalyzed by cobaloximes: Functional models for hydrogenases. Inorg. Chem. 44, 4786–4795 (2005).
Dempsey, J. L., Winkler, J. R. & Gray, H. B. Mechanism of H2 evolution by a photogenerated hydridocobaloxime. J. Am. Chem. Soc. 132, 16774–16776 (2010).
Hu, X. L., Cossairt, B. M., Brunschwig, B. S., Lewis, N. S. & Peters, J. C. Electrocatalytic hydrogen evolution by cobalt difluoroboryl-diglyoximate complexes. Chem. Commun. 4723–4725 (2005).
Hu, X., Brunschwig, B. S. & Peters, J. C. Electrocatalytic hydrogen evolution at low overpotentials by cobalt macrocyclic glyoxime and tetraimine complexes. J. Am. Chem. Soc. 129, 8988–8998 (2007).
Fourmond, V., Jacques, P. A., Fontecave, M. & Artero, V. H2 evolution and molecular electrocatalysts: Determination of overpotentials and effect of homoconjugation. Inorg. Chem. 49, 10338–10347 (2010).
Jaramillo, T. F. et al. Identification of active edge sites for electrochemical H2 evolution from MoS2 nanocatalysts. Science 317, 100–102 (2007).
Reece, S. Y. et al. Wireless solar water splitting using silicon-based semiconductors and earth-abundant catalysts. Science 334, 645–648 (2011).
McKone, J. R. et al. Evaluation of Pt, Ni, and Ni–Mo electrocatalysts for hydrogen evolution on crystalline Si electrodes. Energy Environ. Sci. 4, 3573–3583 (2011).
Sun, Y. et al. Molecular cobalt pentapyridine catalysts for generating hydrogen from water. J. Am. Chem. Soc. 133, 9212–9215 (2011).
Chen, W-F. et al. Hydrogen-evolution catalysts based on non-noble metal nickel–molybdenum nitride nanosheets. Angew. Chem. Int. Ed. 51, 6131–6135 (2012).
Soto, A. B., Arce, E. M., Palomar-Pardave, M. & Gonzalez, I. Electrochemical nucleation of cobalt onto glassy carbon electrode from ammonium chloride solutions. Electrochim. Acta 41, 2647–2655 (1996).
Cui, C. Q., Jiang, S. P. & Tseung, A. C. C. Electrodeposition of cobalt from aqueous chloride solutions. J. Electrochem. Soc. 137, 3418–3423 (1990).
Pantani, O., Anxolabehere-Mallart, E., Aukauloo, A. & Millet, P. Electroactivity of cobalt and nickel glyoximes with regard to the electro-reduction of protons into molecular hydrogen in acidic media. Electrochem. Commun. 9, 54–58 (2007).
Berben, L. A. & Peters, J. C. Hydrogen evolution by cobalt tetraimine catalysts adsorbed on electrode surfaces. Chem. Commun. 46, 398–400 (2010).
Anxolabehere-Mallart, E. et al. Boron-capped tris(glyoximato) cobalt clathrochelate as a precursor for the electrodeposition of nanoparticles catalyzing H2 evolution in water. J. Am. Chem. Soc. 134, 6104–6107 (2012).
Hu, G-R., Deng, X-R., Peng, Z-D. & Du, K. Comparison of AlPO4− and Co3(PO4)2− coated LiNi0.8Co0.2O2 cathode materials for Li-ion battery. Electrochim. Acta 53, 2567–2573 (2008).
Yang, J., Liu, H., Martens, W. N. & Frost, R. L. Synthesis and characterization of cobalt hydroxide, cobalt oxyhydroxide, and cobalt oxide nanodiscs. J. Phys. Chem. C 114, 111–119 (2009).
Kanan, M. W. et al. Structure and valency of a cobalt-phosphate water oxidation catalyst determined by in situ X-ray spectroscopy. J. Am. Chem. Soc. 132, 13692–13701 (2010).
Trasatti, S. Work function, electronegativity, and electrochemical behavior of metals. 3. Electrolytic hydrogen evolution in acid solutions. J. Electroanal. Chem. 39, 163–184 (1972).
Miles, M. H. Evaluation of electrocatalysts for water electrolysis in alkaline-solutions. J. Electroanal. Chem. 60, 89–96 (1975).
Khaselev, O. & Turner, J. A. A monolithic photovoltaic-photoelectrochemical device for hydrogen production via water splitting. Science 280, 425–427 (1998).
Rocheleau, R. E., Miller, E. L. & Misra, A. High-efficiency photoelectrochemical hydrogen production using multijunction amorphous silicon photoelectrodes. Energy Fuels 12, 3–10 (1998).
Yamada, Y. et al. One chip photovoltaic water electrolysis device. Int. J. Hydrogen Energy 28, 1167–1169 (2003).
Sivula, K., Le Formal, F. & Gratzel, M. Solar water splitting: Progress using hematite (α-Fe2O3) photoelectrodes. ChemSusChem 4, 432–449 (2011).
Paracchino, A., Laporte, V., Sivula, K., Gratzel, M. & Thimsen, E. Highly active oxide photocathode for photoelectrochemical water reduction. Nature Mater. 10, 456–461 (2011).
Pijpers, J. J. H., Winkler, M. T., Surendranath, Y., Buonassisi, T. & Nocera, D. G. Light-induced water oxidation at silicon electrodes functionalized with a cobalt oxygen-evolving catalyst. Proc. Natl Acad. Sci. USA 108, 10056–10061 (2011).
Zhong, D. K., Cornuz, M., Sivula, K., Graetzel, M. & Gamelin, D. R. Photo-assisted electrodeposition of cobalt-phosphate (Co-Pi) catalyst on hematite photoanodes for solar water oxidation. Energy Environ. Sci. 4, 1759–1764 (2011).
Young, E. R., Nocera, D. G. & Bulovic, V. Direct formation of a water oxidation catalyst from thin-film cobalt. Energy Environ. Sci. 3, 1726–1728 (2010).
Maeda, K., Higashi, M., Lu, D. L., Abe, R. & Domen, K. Efficient nonsacrificial water splitting through two-step photoexcitation by visible light using a modified oxynitride as a hydrogen evolution photocatalyst. J. Am. Chem. Soc. 132, 5858–5868 (2010).
Maeda, K. et al. Photocatalyst releasing hydrogen from water—enhancing catalytic performance holds promise for hydrogen production by water splitting in sunlight. Nature 440, 295–295 (2006).
Acknowledgements
The authors thank P. Jegou for XPS measurements, B. Sartor for the design and construction of a specific electrochemical cell allowing working with FTO-coated glass electrodes, P. Chernev, K. Klingan, M. Risch and I. Zaharieva (FU Berlin) as co-workers during the XAS measurements at the KMC-1 beamline of the BESSY synchrotron (Helmholtz Zentrum Berlin, HZB) which were technically supported by F. Schäfers and M. Mertin (HZB). Financial support by the Nanosciences Program of CEA (Grant Nanocat’ O2), the UniCat cluster of excellence (Unifying Concepts in Catalysis, Berlin) and the European Commission (7th Framework Programme, SOLAR-H2, grant # 212508) is gratefully acknowledged.
Author information
Authors and Affiliations
Contributions
V.A. and M.F. designed research; S.C., V.F., P-A.J., J.F., V.I. and V.A. performed research; J.H. and H.D. performed XAS studies; B.J. and S.P. performed XPS studies; L.G. and S.C. performed SEM measurements and surface EDX analysis; V.A. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
A European patent application (EP-12352001) has been filed for the preparation, characterization and properties of H2-CoCat.
Supplementary information
Supplementary Information
Supplementary Information (PDF 2115 kb)
Rights and permissions
About this article
Cite this article
Cobo, S., Heidkamp, J., Jacques, PA. et al. A Janus cobalt-based catalytic material for electro-splitting of water. Nature Mater 11, 802–807 (2012). https://doi.org/10.1038/nmat3385
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat3385
This article is cited by
-
On the nature of Con±/0 clusters reacting with water and oxygen
Communications Chemistry (2024)
-
Recent Advances in High-Efficiency Electrocatalytic Water Splitting Systems
Electrochemical Energy Reviews (2023)
-
Keeping sight of copper in single-atom catalysts for electrochemical carbon dioxide reduction
Nature Communications (2022)
-
Electrodeposition, formation mechanism, and electrocatalytic performance of Co-Ni-P ternary catalysts coated on carbon fiber paper
Journal of Solid State Electrochemistry (2021)
-
Electrocatalytic Behavior of Hydrogenated Pd-Metallic Glass Nanofilms: Butler-Volmer, Tafel, and Impedance Analyses
Electrocatalysis (2020)