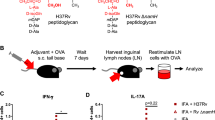
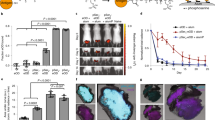
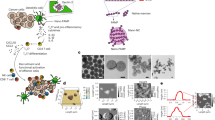
Abstract
Granules of mast cells (MCs) enhance adaptive immunity when, on activation, they are released as stable particles. Here we show that submicrometre particles modelled after MC granules augment immunity when used as adjuvants in vaccines. The synthetic particles, which consist of a carbohydrate backbone with encapsulated inflammatory mediators such as tumour necrosis factor, replicate attributes of MCs in vivo including the targeting of draining lymph nodes and the timed release of the encapsulated mediators. When used as an adjuvant during vaccination of mice with haemagglutinin from the influenza virus, the particles enhanced adaptive immune responses and increased survival of mice on lethal challenge. Furthermore, differential loading of the particles with the cytokine IL-12 directed the character of the response towards Th1 lymphocytes. The synthetic MC adjuvants replicate and enhance the functions of MCs during vaccination, and can be extended to polarize the resulting immunity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Lambrecht, B. N., Kool, M., Willart, M. A. & Hammad, H. Mechanism of action of clinically approved adjuvants. Curr. Opin Immunol. 21, 23–29 (2009).
Martín-Fontecha, A. et al. Regulation of dendritic cell migration to the draining lymph node: Impact on T lymphocyte traffic and priming. J. Exp. Med. 198, 615–621 (2003).
McLachlan, J. B. et al. Mast cell-derived tumor necrosis factor induces hypertrophy of draining lymph nodes during infection. Nature Immunol. 4, 1199–1205 (2003).
Shelburne, C. P. et al. Mast cells augment adaptive immunity by orchestrating dendritic cell trafficking through infected tissues. Cell Host Microbe. 6, 331–342 (2009).
Abraham, S. N. & St. John, A. L. Mast cell-orchestrated immunity to pathogens. Nature Rev. Immunol. 10, 440–452 (2010).
Kunder, C. A. et al. Mast cell-derived particles deliver peripheral signals to remote lymph nodes. J. Exp. Med. 206, 2455–2467 (2009).
McLachlan, J. B. et al. Mast cell activators: A new class of highly effective vaccine adjuvants. Nature Med. 14, 536–541 (2008).
Bradney, C. P., Sempowski, G. D., Liao, H. X., Haynes, B. F. & Staats, H. F. Cytokines as adjuvants for the induction of anti-human immunodeficiency virus peptide immunoglobulin G (IgG) and IgA antibodies in serum and mucosal secretions after nasal immunization. J. Virol. 76, 517–524 (2002).
Perrillo, R. Benefits and risks of interferon therapy for hepatitis B. Hepatology 49, S103–S111 (2009).
Anderlini, P., Przepiorka, D., Champlin, R. & Korbling, M. Biologic and clinical effects of granulocyte colony-stimulating factor in normal individuals. Blood 88, 2819–2825 (1996).
Stevens, R. L. & Adachi, R. Protease–proteoglycan complexes of mouse and human mast cells and importance of their β-tryptase–heparin complexes in inflammation and innate immunity. Immunol. Rev. 217, 155–167 (2007).
Roy, K., Mao, H. Q., Huang, S. K. & Leong, K. W. Oral gene delivery with chitosan–DNA nanoparticles generates immunologic protection in a murine model of peanut allergy. Nature Med. 5, 387–391 (1999).
Kuang, M. et al. Phase II randomized trial of autologous formalin-fixed tumor vaccine for postsurgical recurrence of hepatocellular carcinoma. Clin. Cancer Res. 10, 1574–1579 (2004).
Hanes, J. et al. Controlled local delivery of interleukin-2 by biodegradable polymers protects animals from experimental brain tumors and liver tumors. Pharm. Res. 18, 899–906 (2001).
VandeVord, P. J. et al. Evaluation of the biocompatibility of a chitosan scaffold in mice. J. Biomed. Mater. Res. 59, 585–590 (2002).
Lane, D. A. & Adams, L. Non-anticoagulant uses of heparin. New Engl. J. Med. 329, 129–130 (1993).
Phaechamud, T., Koizumi, T. & Ritthidej, G. C. Chitosan citrate as film former: Compatibility with water-soluble anionic dyes and drug dissolution from coated tablet. Int. J. Pharm. 198, 97–111 (2000).
Hammel, I., Lagunoff, D. & Galli, S. J. Regulation of secretory granule size by the precise generation and fusion of unit granules. J. Cell Mol. Med. 14, 1904–1916 (2010).
Ingulli, E., Ulman, D. R., Lucido, M. M. & Jenkins, M. K. In situ analysis reveals physical interactions between CD11b+ dendritic cells and antigen-specific CD4 T cells after subcutaneous injection of antigen. J. Immunol. 169, 2247–2252 (2002).
Junt, T. et al. Subcapsular sinus macrophages in lymph nodes clear lymph-borne viruses and present them to antiviral B cells. Nature 450, 110–114 (2007).
Phan, T. G., Grigorova, I., Okada, T. & Cyster, J. G. Subcapsular encounter and complement-dependent transport of immune complexes by lymph node B cells. Nature Immunol. 8, 992–1000 (2007).
Klein, U. & Dalla-Favera, R. Germinal centres: Role in B-cell physiology and malignancy. Nature Rev. Immunol. 8, 22–33 (2008).
Villiers, C. et al. From secretome analysis to immunology: Chitosan induces major alterations in the activation of dendritic cells via a TLR4-dependent mechanism. Mol. Cell Proteomics 8, 1252–1264 (2009).
Sui, Z., Chen, Q., Fang, F., Zheng, M. & Chen, Z. Cross-protection against influenza virus infection by intranasal administration of M1-based vaccine with chitosan as an adjuvant. Vaccine 28, 7690–7698 (2010).
Ghendon, Y., Markushin, S., Akopova, I., Koptiaeva, I. & Krivtsov, G. Chitosan as an adjuvant for poliovaccine. J. Med. Virol. 83, 847–852 (2011).
Bhan, A. K., Nadler, L. M., Stashenko, P., McCluskey, R. T. & Schlossman, S. F. Stages of B cell differentiation in human lymphoid tissue. J. Exp. Med. 154, 737–749 (1981).
Nimmerjahn, F. & Ravetch, J. V. Divergent immunoglobulin g subclass activity through selective Fc receptor binding. Science 310, 1510–1512 (2005).
Pullen, G. R., Fitzgerald, M. G. & Hosking, C. S. Antibody avidity determination by ELISA using thiocyanate elution. J. Immunol. Methods 86, 83–87 (1986).
Harris, S. L., Tsao, H., Ashton, L., Goldblatt, D. & Fernsten, P. Avidity of the immunoglobulin G response to a Neisseria meningitidis group C polysaccharide conjugate vaccine as measured by inhibition and chaotropic enzyme-linked immunosorbent assays. Clin. Vaccine Immunol. 14, 397–403 (2007).
Marcus, H. et al. Contribution of immunological memory to protective immunity conferred by a Bacillus anthracis protective antigen-based vaccine. Infect. Immun. 72, 3471–3477 (2004).
McCloskey, N., Turner, M. W. & Goldblatt, T. D. Correlation between the avidity of mouse–human chimeric IgG subclass monoclonal antibodies measured by solid-phase elution ELISA and biospecific interaction analysis (BIA). J. Immunol. Methods 205, 67–72 (1997).
Stetson, D. B. & Medzhitov, R. Type I interferons in host defense. Immunity 25, 373–381 (2006).
Afonso, L. C. et al. The adjuvant effect of interleukin-12 in a vaccine against Leishmania major. Science 263, 235–237 (1994).
Mosca, F. et al. Molecular and cellular signatures of human vaccine adjuvants. Proc. Natl Acad. Sci. USA 105, 10501–10506 (2008).
Datta, S. K. et al. Mucosal adjuvant activity of cholera toxin requires Th17 cells and protects against inhalation anthrax. Proc. Natl Acad. Sci. USA 107, 10638–10643 (2010).
De Gregorio, E., D’Oro, U. & Wack, A. Immunology of TLR-independent vaccine adjuvants. Curr. Opin. Immunol. 21, 339–345 (2009).
Galli, S. J., Nakae, S. & Tsai, M. Mast cells in the development of adaptive immune responses. Nature Immunol. 6, 135–142 (2005).
Liang, M. T., Davies, N. M., Blanchfield, J. T. & Toth, I. Particulate systems as adjuvants and carriers for peptide and protein antigens. Curr. Drug Deliv. 3, 379–388 (2006).
Kasturi, S. P. et al. Programming the magnitude and persistence of antibody responses with innate immunity. Nature 470, 543–547 (2011).
Jewell, C. M., Lopez, S. C. & Irvine, D. J. In situ engineering of the lymph node microenvironment via intranodal injection of adjuvant-releasing polymer particles. Proc. Natl Acad. Sci. USA 108, 15745–15750 (2011).
Singh, M. et al. Cationic microparticles are an effective delivery system for immune stimulatory cpG DNA. Pharm. Res. 18, 1476–1479 (2001).
Acknowledgements
We thank C. A. Kunder, B. Berwin and A. Alonso for their contributions to producing the image in Fig. 1a. L. Eibist is thanked for her assistance in acquiring the EM of synthetic particles. We also thank A. P. S. Rathore and W. X. G. Ang for their manuscript review and discussions. The authors’ work is supported by the US National Institutes of Health grants R01 AI96305, R01 AI35678, R01 DK077159, R01 AI50021, R37 DK50814 and R21 AI056101.
Author information
Authors and Affiliations
Contributions
Experiments were designed by A.L.S. and S.N.A., with H.F.S. contributing to the experimental design of vaccination studies and K.W.L. contributing to the experimental design of studies relating to particle synthesis and characterization. Experiments were carried out by A.L.S. and C.Y.C. Data was analysed by A.L.S. and C.Y.C. with advice from S.N.A. and H.F.S. The manuscript was written primarily by A.L.S. All authors contributed to discussions and manuscript review.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 512 kb)
Rights and permissions
About this article
Cite this article
St. John, A., Chan, C., Staats, H. et al. Synthetic mast-cell granules as adjuvants to promote and polarize immunity in lymph nodes. Nature Mater 11, 250–257 (2012). https://doi.org/10.1038/nmat3222
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat3222
This article is cited by
-
Novel mucosal adjuvant, mastoparan-7, improves cocaine vaccine efficacy
npj Vaccines (2020)
-
Angiogenesis in pancreatic cancer: current research status and clinical implications
Angiogenesis (2019)
-
Exploiting the pliability and lateral mobility of Pickering emulsion for enhanced vaccination
Nature Materials (2018)
-
Designing nanomedicine for immuno-oncology
Nature Biomedical Engineering (2017)
-
From sewer to saviour — targeting the lymphatic system to promote drug exposure and activity
Nature Reviews Drug Discovery (2015)