Abstract
Protective immunity against Mycobacterium tuberculosis involves major histocompatibility complex class I (MHC-I)- and CD1-restricted CD8 T cells, but the mechanisms underlying antigen delivery to antigen-presenting molecules remain enigmatic. Macrophages, the primary host cells for mycobacteria, are CD1-negative. Here we show that M. tuberculosis phagosomes are secluded from the cytosolic MHC-I processing pathway and that mycobacteria-infected cells lose their antigen-presenting capacity. We also show that mycobacteria induce apoptosis in macrophages, causing the release of apoptotic vesicles that carry mycobacterial antigens to uninfected antigen-presenting cells (APCs). Inhibition of apoptosis reduced transfer of antigens to bystander cells and activation of CD8 T cells. Uninfected dendritic cells, which engulfed extracellular vesicles, were indispensable for subsequent cross-presentation of antigens, through MHC-I and CD1b, to T cells from mycobacteria-sensitized donors. This new 'detour' pathway for presentation of antigens from a phagosome-contained pathogen shows the functional significance of infection-induced apoptosis in the activation of CD8 T cells specific for both protein and glycolipid antigens in tuberculosis.
Similar content being viewed by others
Main
Tuberculosis remains a major global health threat, claiming more lives than any other bacterial infection1. The etiologic agent, M. tuberculosis, survives within phagosomes of macrophages and persists even after T-cell immunity has been established. A major survival strategy of this pathogen is the arrest of phagosome maturation toward the phagolysosome stage2. This early endosomal habitat shields the pathogen from host effector mechanisms and secludes its antigens from processing pathways. Although CD4 T cells have a central role in immunity to M. tuberculosis, other T-cell populations contribute to protection against this pathogen, including MHC-I-restricted CD8 T cells and T cells restricted by CD1a, CD1b and CD1c (group I CD1 molecules)3,4,5,6,7. The role of CD8 T cells in protection against tuberculosis has been established by infection experiments in mice lacking CD8 T cells4,7. Not only do these T cells produce interferon (IFN)-γ for activation of macrophages, but they can also kill mycobacteria upon lysis of the infected host cell3,5,8. In contrast to conventional T cells, which recognize antigenic peptides, group I CD1-restricted T cells respond to mycobacterial glycolipids such as glucose monomycolate, lipoarabinomannan (LAM) and isoprenoids5,6. The mechanisms underlying antigen delivery to the CD1 and MHC-I pathways remain unresolved. Macrophages, the primary host cells for mycobacteria, are CD1-negative; CD1 molecules are mainly expressed on dendritic cells. These cells, which also express high levels of MHC-I, MHC–II and costimulatory molecules, represent the key APCs for the induction of a specific T-cell response. Based on in vitro studies, a membranolytic activity similar to that of Listeria monocytogenes has been postulated for M. tuberculosis9. This would allow antigen delivery into the cytosol, where classical MHC-I antigen processing takes place. However, we provide compelling evidence that M. tuberculosis does not penetrate the phagosomal membrane to access the cytosolic compartment, but intracellular mycobacteria released antigens that were transferred within apoptotic vesicles to uninfected bystander APCs. Subsequently, bystander APCs presented mycobacterial antigens to CD8 T cells through MHC-I and CD1b. Transfer of antigens from infected cells to potent APCs such as dendritic cells represents an alternative pathway, involving infection-mediated apoptosis of the host cell, by which CD8 T cells can recognize antigens of the phagosomally secluded tubercle bacillus.
Results
M. tuberculosis phagosomes are secluded from the cytoplasm
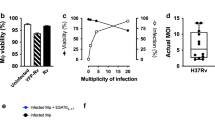
CD8 T cells are activated by M. tuberculosis in mice and humans, raising the question of how mycobacterial antigens are delivered to the respective processing and presentation pathways. We found that membrane-impermeant fluorescent molecules (8-hydroxypyrene-1,3,6-trisulfonic acid (HPTS)10, dextran–Texas Red and LLO91-99-FITC) engulfed by macrophages together with M. tuberculosis did not leak out of the phagosome into the host cell cytoplasm for up to 48 h after infection (Fig. 1a,b). Experiments were not extended to later time points because infected cells succumbed to apoptosis at 48 h after infection (see below). These dyes were retained in phagosomes labeled for either the early (transferrin) or late (ovalbumin) endosomal and lysosomal stages11. Thus, mycobacterial phagosomes did not access the host cell's cytoplasm independently of their maturation stage (Fig. 1c). In contrast, these dyes were released from the L. monocytogenes phagosome early during phagosome maturation (<10 min after infection; Fig. 1a)10. Moreover, upon infection of macrophages labeled with mycobacteria containing [14C]amino acids, < 4% of the total radioactivity was detected in the cytosolic fraction within 48 h (Fig. 1d). Although we cannot formally exclude transfer of antigenic material into the cytosol, such a small amount of radioactivity was probably released during cell lysis, and the vast majority was retained in the phagosomes. Thus, the membrane of the mycobacterial phagosome was virtually impermeable to macromolecules, including peptides (Fig. 1b). This is corroborated by recent electron microscopy studies showing that the phagosomal membrane remains intact during infection12,13. Microinjected dextran was found in close association with bacillus Calmette-Guérin (BCG) phagosomes14. BCG is less efficient at blocking phagolysosome fusion, however, and therefore may intersect with the host cell autophagous system for cytoplasmic material, as shown for late endosome-like leishmania phagosomes15.
(a) Mouse macrophages were incubated with M. tuberculosis or L. monocytogenes in the presence of HPTS. HPTS transfer was followed in living cells (arrows indicate bacteria-containing phagosomes). (b) Mouse macrophages were infected with M. tuberculosis in the presence of LLO91-99-FITC and stained using a BCG-specific antibody (rabbit anti-BCG). (c) Phagosomes (arrows) are not leaky for coloaded tracers even at 48 h after infection. Mouse macrophages infected with M. tuberculosis in the presence of Ova-Cy3 (red) were incubated for 48 h. Late endosomes and lysosomes or early endosomes were labeled using Ova-Cy2 or transferrin-FITC (green), respectively11. Confocal laser scanning microscopy revealed that both phagolysosomes and early phagosomes, as indicated by yellow fluorescence, retain the coloaded tracer. (d) Mycobacteria do not release significant amounts of material into the cytosol. Shown is the mean percentage of cytosolic versus total radioactivity from macrophages infected with BCG [14C]amino acids (three measurements from one representative experiment out of three).
Mycobacteria release antigens for transport to bystander cells
Seclusion of mycobacteria from the classical MHC-I processing pathway (from cytoplasm to endoplasmic reticulum) suggests an alternative path for delivery of mycobacterial antigens to MHC-I molecules16,17. Phagosomal mycobacteria release antigens that are transported to lysosomes and uninfected bystander APCs11,18,19,20,21. We purified extracellular vesicles from the culture supernatants of mycobacteria-infected APCs by differential centrifugation. Extracellular vesicles from APCs (macrophages and dendritic cells) infected with live Alexa 568–hydrazide-labeled mycobacteria transferred, upon coculture, labeled glycolipids to uninfected APCs (Fig. 2a)11. We did not observe glycolipid transfer upon infection with labeled, heat-killed mycobacteria (Fig. 2a). The main cell wall lipids labeled by Alexa 568–hydrazide were LAM and phosphatidyl-inositol mannosides (PIMn)11. To define the nature of the mycobacterial material in extracellular vesicles, mycobacteria were labeled with either [14C]palmitic acid for lipids and lipoproteins or [14C]amino acids for proteins. Extracellular vesicles from APCs infected with labeled, heat-killed mycobacteria did not transfer significant amounts of antigen (Fig. 2b; data not shown). Extracellular vesicles, phagosomes and lysosomes were purified from infected APCs and analyzed for antigenic content. Extracellular vesicles from APCs infected with [14C]palmitic acid–labeled mycobacteria contained several mycobacterial lipids, including mycoside B, lysocardiolipin and PIMn, which were also present in lysosomes and phagosomes (Fig. 2b)11,18,20. Vesicles from APCs infected with mycobacteria labeled with [14C]amino acids carried proteins in the molecular mass range below 38 kDa, which was lower than that of most proteins present in lysosomes and phagosomes (Fig. 2c). These low molecular mass proteins were also detected using a polyclonal rabbit antiserum to BCG, but not by a preimmune rabbit serum (Fig. 2c). The band of approximately 18 kDa stained with an antibody specific for the p19 lipoprotein (data not shown)21. Extracellular vesicles also contained CD1b, the macrophage mannose receptor and MHC-II, but not the early endosomal antigen-1 or the late endosomal and lysosomal marker cathepsin D (Fig. 2c; data not shown). Thus, extracellular vesicles drag along APC-derived plasma membrane proteins. Electron microscopy of extracellular vesicles from mycobacteria-infected APCs revealed a heterogeneous population consisting mainly of large vesicles reminiscent of apoptotic blebs, multilamellar bodies and smaller vesicles resembling exosomes (Fig. 2d)18,22,23. Immunoelectron microscopy using specific monoclonal antibodies to mycobacterial antigens detected trehalose dimycolate (TDM), PIMn, LAM and p19 in these vesicles (Fig. 2e,f).
(a) Extracellular vesicles purified from human macrophages were treated with either live or heat-killed (HK) BCG labeled with Alexa 568–hydrazide, and were incubated with uninfected CFDA-SE–labeled dendritic cells (green). Engulfed cell wall material is shown in red. (b) Left three panels show two-dimensional HPTLC analysis of lipid extracts from phagosomes, lysosomes and extracellular vesicles from macrophages infected with [14C]palmitic acid–labeled BCG. Positions of the respective lipids were determined using known standards: lysocardiolipin (LCL), mycoside B (MycB), phosphatidylethanolamine (PE), phosphatidylglycerol (PG); phosphatidylinositol (PI), PIMn and TDM20. Extracellular vesicles from comparable numbers of macrophages infected with either live or heat-killed [14C]palmitic acid–labeled BCG were separated by one-dimensional HPTLC (second panel from right) or measured for radioactivity (far right panel; c.p.m., counts per minute), revealing that almost no radioactivity was released from cells infected with dead mycobacteria (*, P < 0.05). (c) Proteins from phagosomes (Phag), lysosomes (Lys) and extracellular vesicles (Ex) from macrophages infected with mycobacteria containing [14C]amino acids were separated by SDS-PAGE and analyzed by phosphoimager (left) or western blotting using a rabbit polyclonal BCG-specific antiserum [middle) and rabbit preimmune serum (middle, last lane), or antibodies to CD1b or cathepsin D (right). (d) Electron microscopy of vesicles from APCs infected with mycobacteria, showing morphological heterogeneity. Scale bar = 2 μm. (e) Immunoelectron microscopy of extracellular vesicles from macrophages infected with M. bovis BCG, using a monoclonal antibody to TDM (anti TDM). Control was an irrelevant monoclonal antibody. (f) Quantification of gold labeling for LAM, PIMn, TDM and p19 in extracellular vesicles from infected macrophages, compared with background labeling. Gold particle counts are shown as mean ± s.d. from eight or nine independent pictures.
Antigen transfer by apoptotic vesicles for CD8 T-cell activation
To analyze the source of the extracellular vesicles from mycobacteria-infected APCs, we used annexin-V as a marker for phosphatidylserine exposure on the membranes of apoptotic cells. This analysis, together with electron microscopy, showed that APCs (macrophages and dendritic cells of both human and mouse origin) infected with M. tuberculosis underwent programmed cell death and released or disintegrated into apoptotic vesicles by 48 h after infection (Fig. 3a,b). Cells also became apoptotic at 48 h after BCG infection, but at a lower frequency (∼25%). Apoptosis was not seen in uninfected APCs (Fig. 3a,b). Treatment of infected cells with the caspase inhibitor zVADfmk, which inhibits apoptotic blebbing24, blocked transfer of Alexa 568–hydrazide-labeled cell wall material through extracellular vesicles to uninfected dendritic cells (Fig. 4a). Hence, the majority of transferred antigens were shuttled by apoptotic blebs. Moreover, transfer of cell wall material from infected to uninfected APCs was inhibited by treatment of recipient cells with RGD-peptide (55%), soluble CD14 (40%) or cytochalasin D (>90%; Fig. 4a; data not shown). RGD specifically blocks uptake of apoptotic blebs or cells through the cell binding site of β3 integrins such as the vitronectin receptor; CD14 serves as a receptor for apoptotic blebs or cells and cytochalasin D inhibits actin-based engulfment25,26,27. Extracellular vesicles engulfed by uninfected APCs were transported within 1 h of observation into compartments that were positive for the late endosomal and lysosomal tracer, mannosylated BSA-FITC (30-min pulse, 2-h chase), or lysosome-associated membrane protein-1 (Fig. 4b; data not shown).
(a) Apoptosis of infected, but not uninfected, APCs was detected by annexin-V (Annexin-V-Biot). Higher magnification (far right panels) shows infected cells disintegrating into annexin-V-positive apoptotic blebs. Apoptotic dendritic cells are attached to adherent cells. SAV-Cy2, streptavidin-Cy2. (b) Electron microscopy of M. tuberculosis–infected macrophages or dendritic cells (black arrows indicate mycobacterial phagosomes) shows shedding of extracellular vesicles (white arrows indicate extracellular vesicles). General morphological appearance changes at 48 h after infection, with membrane blebbing all over the cell surface and an increase in intracellular multilamellar bodies. Scale bar = 5 μm, except in lower right panel (1 μm).
(a) Extracellular vesicles from macrophages infected with mycobacteria labeled with Alexa 568–hydrazide were added to uninfected dendritic cells. Transfer of label was blocked by treatment of macrophages with the caspase inhibitor zVADfmk, or partially upon incubation of the dendritic cells with RGD or soluble CD14 (sol.). Anti CD14, antibody to CD14; mAb, monoclonal antibody; SIINFEKL, control peptide (b) Pkh26-labeled extracellular vesicles (Ex-Pkh26; red) are transported to late endosomes and lysosomes labeled with mannosylated BSA-FITC (man BSA; green). Yellow fluorescence indicates colocalization ('Merge' column). (c) Proliferation of CD8 T cells (PPD-positive donor; TC) upon coculture with autologous dendritic cells (DC) in the presence of untreated or M. tuberculosis–infected macrophages (*, P < 0.05). (d) The capability of macrophages to activate CD8 T cells (PPD-positive donor) is decreased after M. tuberculosis infection (*, P < 0.05). (e) Proliferation of CD8 T cells (PPD-positive donor) upon coculture with dendritic cells in the presence of untreated or M. tuberculosis–infected macrophages. Inhibition of apoptotic bleb formation in infected macrophages by zVADfmk (VAD) blocked T-cell activation (*, P < 0.05). T cells responded normally to SEB. Shown are means ± s.d. from triplicate measurements from one representative experiment out of three.
To assess the relevance of apoptosis in the activation of mycobacteria-specific CD8 T cells, CD8 T cells from purified protein derivative (PPD)-positive donors were cultured together with uninfected autologous dendritic cells in the presence of untreated macrophages or macrophages infected with M. tuberculosis for 6 d. T-cell proliferation depended on addition of uninfected bystander dendritic cells to the cultures and was only observed in the presence of infected macrophages (Fig. 4c). Consistent with this observation, macrophages infected with M. tuberculosis lost their capacity to present antigens to CD8 T cells within 2 d of infection (Fig. 4d). In contrast, pretreatment of M. tuberculosis–infected macrophages with zVADfmk, before T cells and dendritic cells were added, abrogated the CD8 T-cell response to bystander dendritic cells cocultured with infected macrophages (Fig. 4e). However, zVADfmk-treated T cells still responded to staphylococcal enterotoxin B (SEB) or phytohemagglutinin (PHA), confirming no direct effect by this treatment (Fig. 4e; data not shown). We conclude that extracellular vesicles derived from M. tuberculosis-infected macrophages undergoing cell death targets antigens to proficient, uninfected bystander dendritic cells for presentation to CD8 T cells.
Antigen targeting for MHC-I- and CD1b-mediated T cell activation
To examine the importance of the detour pathway (involving extracellular vesicles) in CD8 T-cell activation, we stimulated CD8 T cells from PPD-negative or PPD-positive healthy donors (Fig. 5a,b) or tuberculosis patients (Fig. 5c) with autologous dendritic cells pulsed with extracellular vesicles from mycobacteria-infected APCs. These CD8 T cells responded by proliferating and secreting IFN-γ only when vesicles from infected cells were used (Fig. 5a–c; PHA-response: PPD+/11,065 (±795) c.p.m., PPD-/8,200 (±1,228) c.p.m.). Fluorescence-activated cell sorting (FACS) showed that CD8 T cells only expanded in the presence of extracellular vesicles from infected APCs (Fig. 5b). Extracellular vesicles from autologous and heterologous infected APCs activated T cells equally well (Fig. 5b). This, together with the findings that mycobacteria-infected macrophages, which disintegrate into apoptotic blebs, were inefficient in antigen presentation in the absence of bystander dendritic cells (Fig. 4c), and that extracellular macrophage-derived vesicles alone did not activate T cells (data not shown), implies that antigen-presenting molecules on recipient APCs, rather than those on extracellular vesicles, are responsible for presentation. No response was seen with CD8 T cells from PPD-negative donors, which nevertheless responded normally to a polyclonal stimulus (PHA; Fig. 5a). Proliferation of CD8 T cells was specifically blocked by MHC-I- and CD1b-specific monoclonal antibodies by >90 % and ∼20%, respectively, but not by CD1a- or CD1c-specific antibodies (Fig. 5a). IFN-γ production was reduced by 70–80% and by 30–50% after blocking of MHC-I or CD1b, respectively (Fig. 5b). CD8 T cells from patients hospitalized because of clinical tuberculosis, despite higher background activation, responded specifically (proliferation and IFN-γ production in six of eight patients tested) to extracellular vesicles from mycobacteria-infected macrophages (Fig. 5c). To more precisely examine the processing pathway of antigen-containing extracellular vesicles, we used bafilomycin, an inhibitor of the vesicular H+ ATPase, to block acidification of late endosomes, lysosomes and lysosomal enzymes, and MG132, a proteasome inhibitor. Inhibition of proteasomes had a small but dose-dependent effect on antigen presentation, but was not statistically significant (Fig. 5d). In contrast, inhibition of endosomal acidification almost completely blocked presentation of mycobacterial antigens from extracellular vesicles to CD8 T cells. Thus, mycobacterial antigens delivered by extracellular vesicles to bystander cells require an intact, acidified late endosomal and lysosomal compartment for presentation by dendritic cells.
(a) Proliferation of CD8 T cells (TC) from PPD-positive (▪) and PPD-negative donors (□) upon coculture with autologous dendritic cells (DC) pulsed with extracellular vesicles from infected (Ex-inf) or noninfected (Ex-ni) APCs. (b) Dose-dependent IFN-γ production of CD8 T cells (PPD-positive donor) stimulated with autologous dendritic cells pulsed with different concentrations of extracellular vesicles from heterologous APCs (1×, 70 μg/ml protein). a,b, We used blocking antibodies (anti-CD1a, anti-CD1b, anti-CD1c and anti-MHC) in both assays. Shown are means ±s.d. of triplicate measurements from one representative experiment done with cells from one donor (n = 6). *, P < 0.01; **, P < 0.001. FACS analysis (upper right) shows predominant expansion of CD8 T cells. (c) CD8 T cells from tuberculosis (Tb) patients proliferate and produce IFN-γ in response to extracellular vesicles from mycobacteria-infected or noninfected APCs, presented by autologous dendritic cells. Data shown are from two of six patients. *, P < 0.01; **, P < 0.001. α-MHC-I, antibody to MHC-I. (d) Presentation of extracellular vesicle–derived mycobacterial antigens by dendritic cells treated with bafilomycin (Bafilo) or MG132. Shown is one representative experiment out of three. **, P < 0.001. (e) Proliferation and IFN-γ secretion in the CD1b-restricted T-cell line LCD4.6 upon stimulation with CD1+ APCs and M. tuberculosis extract or extracellular vesicles from noninfected or mycobacteria-infected macrophages. CD1b restriction was evaluated using a CD1b-blocking antibody (bottom). Shown are means ± s.d. of triplicate measurements from one representative experiment out of three.
The minor contribution of CD1b-restricted T cells to the responses of bulk cultures is consistent with the small proportion of this T-cell population in the peripheral blood5. To verify the relevance of antigen delivery by extracellular vesicles in CD1b-mediated T-cell responses, we used a CD1b-restricted T-cell line specific for mycolic acid from M. tuberculosis28,29. This T-cell line responded in a dose-dependent manner, with proliferation and IFN-γ production, to extracellular vesicles from infected APCs but not to those from uninfected cells (Fig. 5d). These responses were completely blocked by the addition of an antibody to CD1b (Fig. 5d)30. These data also corroborate the findings by high-performance thin-layer chromatography (HPTLC) and immunoelectron microscopy that mycolic acid or antigenically related structures such as TDM are present in extracellular vesicles from infected cells (Fig. 2b,e)28. We conclude that extracellular vesicles from mycobacteria-infected APCs carry antigens to bystander dendritic cells for presentation to T cells by MHC-I and CD1b.
Discussion
Our experiments indicate that upon infection with mycobacteria, APCs release extracellular vesicles during their progression towards apoptotic cell death. These vesicles carry enclosed mycobacterial antigens (lipids as well as proteins) to uninfected bystander APCs, thus promoting presentation to MHC-I- and CD1b-restricted T cells. This alternative pathway for the activation of CD8 T cells is biologically significant, as small molecules including peptides did not traverse from the phagosome into the cytoplasm of the host cell (Fig. 1). Although transfer of minute amounts of mycobacterial material cannot be formally excluded, the vast majority of mycobacterial antigens are virtually secluded from the classical MHC-I processing pathway in the cytoplasm. Moreover, our data show that macrophages lose their capacity to present mycobacterial antigens and to activate CD8 T cells immediately after infection with M. tuberculosis. Macrophages, the primary host cells infected with mycobacteria in vivo, do not, a priori, express appreciable amounts of CD1a, CD1b and CD1c5,17. Although these molecules are expressed by dendritic cells, which can serve as host cells for mycobacteria, expression of CD1b is also downregulated after mycobacterial infection, and infected dendritic cells become less potent in inducing T-cell responses31,32.
The addition of uninfected dendritic cells to mycobacteria-infected macrophage cultures was indispensable for potent stimulation of CD8 T cells. Dendritic cells express high levels of MHC, CD1 and costimulatory molecules for optimal T-cell priming, and possess a lysosome-to-cytosol antigen delivery pathway33,34. We observed delivery of extracellular vesicles into late endosomal and lysosomal compartments of bystander APCs. The integrity of these compartments is a prerequisite for presentation of antigenic cargo from extracellular vesicles, whereas proteasomal processing seems to be of minor importance. Ultimately, activated CD8 T cells secrete IFN-γ, which is central in the control of mycobacterial growth through activation of macrophages. IFN-γ, though ineffective in activating cells that are already infected with mycobacteria, promotes antimycobacterial effector mechanisms in uninfected bystander macrophages17.
The finding that apoptotic vesicles serve as a source of mycobacterial antigens for presentation by bystander APCs, to induce MHC-I- and CD1b-restricted T cells, establishes a new function for apoptosis in tuberculosis. During infection with M. tuberculosis, apoptosis has been observed in a variety of host cells and has also been observed in tuberculous lesions35,36,37. These results, together with findings that only those Salmonella strains that induce apoptosis stimulate CD8 T cells in mice38, provide compelling evidence that cross-priming by extracellular vesicles is a vital prerequisite for CD8 T-cell-dependent host defense against intracellular bacteria, both in mice and humans. This is in agreement with M. tuberculosis being superior to BCG in terms of both induction of apoptosis (Fig. 3a) and stimulation of CD8 T cells1.
As shown in other experimental systems, such as antitumor immunity, apoptotic cells and vesicles thereof are an important source of antigens for MHC-I-mediated cross-presentation by dendritic cells27,39. Our data shed new light on infection-induced apoptosis by adding a significant new function to this physiological process, namely the allocation of microbial antigens to bystander APCs for T-cell stimulation. In addition to apoptotic blebs, small extracellular vesicles, called exosomes, from infected macrophages also contain mycobacterial glycolipid antigens and may therefore also participate in antigen delivery to bystander cells18. Exosomes derived from peptide-pulsed dendritic cells have been successfully used to vaccinate mice against tumors23.
Taken together, this is the first report showing that phagosome-enclosed M. tuberculosis stimulates inflammatory, IFN-γ-secreting CD8 T cells by inducing in their host cells apoptotic blebs that deliver antigens to both the MHC-I and CD1 processing and presentation pathways of bystander APCs. We consider infection-induced cell death to be a crucial step in cross-priming of CD8 T cells in tuberculosis. Elucidation of this new detour pathway for antigens to MHC-I and CD1 can explain how M. tuberculosis activates a broad spectrum of T cells despite being secluded within the early phagosomes of macrophages. Our data will provide guidance for the rational design of new vaccination strategies against this pathogen, such as inclusion of apoptosis-inducing properties40 to target, in addition to CD4 T cells, MHC-I- and CD1-restricted CD8 T cells.
Methods
Bacteria.
M. tuberculosis (Erdman) and Mycobacterium bovis BCG (Copenhagen) grown in 7H9 medium and L. monocytogenes (EGD) in brain-heart infusion were used to infect cells at a multiplicity of infection of five.
Labeling of bacteria and cells.
Mycobacteria were labeled with Alexa 568–hydrazide (Molecular Probes), [14C]palmitic acid or a [14C]amino acid mix (NEN); APCs were labeled with carboxyfluorescein-diacetate-succinimidylester (CFDA-SE; Molecular Probes) as described11,20. Phagosomes, lysosomes and extracellular vesicles purified from APCs were separated by SDS-PAGE, blotted and probed with antibodies or extracted in chloroform and methanol and separated by HPTLC as described11,20. Vesicular and cytosolic material from macrophages infected with radioactively labeled mycobacteria was separated from lysed cells by centrifugation at 1,800 g for 15 min and 100,000 g for 1 h.
Antibodies, tracers, inhibitors and microscopy.
We used antibodies to cathepsin D (Dako), macrophage mannose receptor (PharMingen), early endosomal antigen-1 (Transduction Laboratories), lysosome-associated membrane protein-1 (ID4B; Developmental Hybridoma Bank) and MHC-II (L238; American Type Culture Collection); rabbit BCG-specific antiserum (Dako); rabbit preimmune serum; peroxidase-labeled secondary antibodies (Jackson Laboratories); and rabbit CD1b-specific antiserum (kindly provided by M. Sugita, Harvard Medical School). Enhanced chemiluminescence was used for detection (NEN).
HPTS (100 mM), dextran–Texas Red (100 μg/ml; Molecular Probes) and mannosylated BSA-FITC (50 μg/ml; Sigma) were applied in Ringer solution. Cy2- and Cy3-labeled ovalbumin (Ova-Cy2 and Ova-Cy3, respectively; 100 μg/ml) and transferrin-FITC (5 μg/ml) were prepared as described11. LLO91-99-FITC (MHC-I epitope of L. monocytogenes listeriolysin) was kindly provided by H. Wendschuh (Jerini). HPTS, dextran–Texas Red, Ova-Cy3 and LLO91-99-FITC were used for coloading into phagosomes, together with mycobacteria. Ova-Cy2 (fluid-phase uptake; 2-h pulse, 16-h chase) and mannosylated BSA-FITC (mannose receptor–mediated uptake; 30-min pulse, 2-h chase) were used to label late endosomes and lysosomes; transferrin-FITC (15 min) was used to label early endosomes11. Cells were cultured on cover slips. Upon binding of the bacteria to the cells in the cold (4 °C), dyes were added for 5 min at 37 °C. Cover slips were washed, sealed to slides and observed on a heated microscope stage (Leica DMIRB) under low-light conditions for up to 60 min. For longer time periods, cover slips were incubated at 37 °C in 7% CO2, fixed and embedded in Mowiol for confocal laser scanning microscopy (Leica TCS-NT). Annexin-V-biotin and streptavidin-Cy2 (Becton Dickinson) were used according to the manufacturer's protocol. The concentrations of the inhibitors were 50 μM for zVADfmk, 10 μg/ml for RGD, SIINFEKL (Bachem) and antibodies, and 5 μg/ml for soluble CD14 (kindly provided by R.S. Jack, University of Greifswald). Uptake was estimated by counting cells from five different microscopic fields that were positive for label. APC membranes were labeled with Pkh26 according to the manufacturer's protocol (Sigma).
Macrophages and dendritic cells.
Monocytes from peripheral blood were isolated by consecutive Ficoll and Percoll gradients, adhered to cell culture dishes in PBS for 2 h and cultured for 2 d in RPMI with 10% FCS. Dendritic cells were generated using recombinant human IL-4 and GM-CSF (200 U/ml; Preprotech)11. Mouse macrophages were generated from bone marrow cells of C57BL/6 mice15.
Extracellular vesicles.
Extracellular vesicles were purified from culture supernatants of infected APC by consecutive centrifugations at 800 g, 1,800 g, 25,000 g and 100,000 g. High-speed pellets were free of mycobacteria as verified by culture. Phagosomes and lysosomes were purified as described15,20. Samples were fixed in 4% paraformaldehyde and 2.5% glutaraldehyde and embedded in Spurr resin according to the manufacturer's protocol, and sections were contrasted using lead citrate and analyzed in a Zeiss EM10 microscope. Immunoelectron microscopy was done on cryosections using monoclonal antibodies to TDM (T1.8), LAM (CS35), PIMn (3D6) and p19, and secondary reagents labeled with 10-nm gold. Antibodies were kind gifts from D.G. Russell (Cornell University), J. Belisle (Colorado State University), M. Ehlers (University of Cape Town) and D.B. Young (Imperial College London).
T-cell assays.
This study was approved by the ethics committee of the Free-University Berlin and informed consent was obtained from donors. Peripheral blood lymphocytes from PPD–negative and PPD-positive donors and tuberculosis patients (Asklepios Clinic, Gauting, Munich) were enriched for CD8 T-cells by CD4-specific negative selection on magnetic cell sorting (MACS) columns (Miltenyi Biotec). T cells (5 × 104) were cultured together with irradiated autologous dendritic cells (5 × 104; 5,000 rad) and extracellular vesicles isolated from autologous or heterologous macrophages, for 3 d in triplicate microtiter wells at 37 °C in 5% CO2. Uninfected or mycobacteria-infected macrophages were cultured for 1–6 d. We added zVADfmk on days 2 or 4 before adding T cells and dendritic cells. Extracellular vesicles were purified from either uninfected or M. bovis BCG–infected macrophages cultured for 6 d in the absence of FCS. FCS deprivation induced apoptotic blebs in both groups of APCs. Vesicle preparations were adjusted to a protein content of 70 μg/ml. T cells were pulsed with [3H]thymidine (1 μCi per well for 18 h) for liquid scintillation counting. Blocking antibodies (American Type Culture Collection, or kind gifts from S. Porcelli, Albert Einstein College for Medicine) specific for CD1a (OKT6), CD1b (BCD1b3.1), CD1c (F10/21A3) or MHC-I (WG132; all 10 μg/ml) were added 1 h before addition of T cells. Positive controls were PHA (1 μg/ml; Sigma) or SEB (Sigma). MG132 (0.2 or 0.05 μM) and bafilomycin (0.05 μM; Calbiochem) were added to the APCs 1 h before addition of extracellular vesicles. The compounds were nontoxic at these concentrations. Flow cytometry (FACSCalibur, Becton Dickinson) was done using monoclonal antibodies to CD4 (TT1) and CD8 (GN11/134.D7; Becton Dickinson). The CD1b-restricted T-cell line LCD4.6 (105 cells per well; refs. 28,29) was cultured with 105 CD1+ APCs in the presence of M. tuberculosis extract or extracellular vesicles, and [3H]thymidine uptake and IFN-γ release were measured at 24 h (refs. 29,30).
Statistics.
Tukey's multiple-comparison test was used to compare individual data groups or columns.
References
Kaufmann, S.H. Is the development of a new tuberculosis vaccine possible? Nat. Med. 6, 955–960 (2000).
Russell, D.G., Sturgill-Koszycki, S., Vanheyningen, T., Collins, H.L. & Schaible, U.E. Why intracellular parasitism need not be a degrading experience for Mycobacterium. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 352, 1303–1310 (1997).
Cho, S. et al. Antimicrobial activity of MHC class I-restricted CD8+ T cells in human tuberculosis. Proc. Natl. Acad. Sci. USA 97, 12210–12215 (2000).
Behar, S.M., Dascher, C.C., Grusby, M.J., Wang, C.R. & Brenner, M.B. Susceptibility of mice deficient in CD1d or TAP1 to infection with Mycobacterium tuberculosis. J. Exp. Med. 189, 1973–1980 (1999).
Porcelli, S.A. & Modlin, R.L. The CD1 system: antigen-presenting molecules for T cell recognition of lipids and glycolipids. Annu. Rev. Immunol. 17, 297–329 (1999).
Moody, D.B. et al. CD1c-mediated T-cell recognition of isoprenoid glycolipids in Mycobacterium tuberculosis infection. Nature 404, 884–888 (2000).
Rolph, M.S. et al. MHC class Ia-restricted T cells partially account for beta2-microglobulin-dependent resistance to Mycobacterium tuberculosis. Eur. J. Immunol. 31, 1944–1949 (2001).
Stenger, S. et al. An antimicrobial activity of cytolytic T cells mediated by granulysin. Science 282, 121–125 (1998).
Mazzaccaro, R.J. et al. Major histocompatibility class I presentation of soluble antigen facilitated by Mycobacterium tuberculosis infection. Proc. Natl. Acad. Sci. USA 93, 11786–11791 (1996).
Beauregard, K.E., Lee, K.D., Collier, R.J. & Swanson, J.A. pH-dependent perforation of macrophage phagosomes by listeriolysin O from Listeria monocytogenes. J. Exp. Med. 186, 1159–1163 (1997)
Schaible, U.E., Hagens, K., Fischer, K., Collins, H.L. & Kaufmann, S.H.E. Intersection of group I CD1 molecules and mycobacteria in different intracellular compartments of dendritic cells. J. Immunol. 164, 4843–4852 (2000).
Clemens, D.L. & Horwitz, M.A. Characterization of the Mycobacterium tuberculosis phagosome and evidence that phagosomal maturation is inhibited. J. Exp. Med. 181, 257–270 (1995).
Xu, S. et al. Intracellular trafficking in Mycobacterium tuberculosis and Mycobacterium avium-infected macrophages. J. Immunol. 153, 2568–2576 (1994).
Teitelbaum, R. et al. Mycobacterial infection of macrophages results in membrane-permeable phagosomes. Proc. Natl. Acad. Sci. USA 96, 15190–15195 (1999).
Schaible, U.E. et al. Parasitophorous vacuoles of Leishmania mexicana acquire macromolecules from the host cell cytosol via two independent routes. J. Cell Sci. 112, 681–693 (1999).
Canaday, D.H. et al. Activation of human CD8+ alpha beta TCR+ cells by Mycobacterium tuberculosis via an alternate class I MHC antigen-processing pathway. J. Immunol. 162, 372–379 (1999).
Schaible, U.E., Collins, H.L. & Kaufmann, S.H. Confrontation between intracellular bacteria and the immune system. Adv. Immunol. 71, 267–377 (1999).
Beatty, W.L. et al. Trafficking and release of mycobacterial lipids from infected macrophages. Traffic 1, 235–247 (2000).
Beatty, W.L. & Russell, D.G. Identification of mycobacterial surface proteins released into subcellular compartments of infected macrophages. Infect. Immun. 68, 6997–7002 (2000).
Fischer, K. et al. Mycobacterial lysocardiolipin is exported from phagosomes upon cleavage of cardiolipin by a macrophage-derived lysosomal phospholipase A2 . J. Immunol. 167, 2187–2192 (2001).
Herrmann, J.L., O'Gaora, P., Gallagher, A., Thole, J.E. & Young, D.B. Bacterial glycoproteins: a link between glycosylation and proteolytic cleavage of a 19 kDa antigen from Mycobacterium tuberculosis. EMBO J. 15, 3547–3554 (1996).
Denzer, K., Kleijmeer, M.J., Heijnen, H.F., Stoorvogel, W. & Geuze, H.J. Exosome: from internal vesicle of the multivesicular body to intercellular signaling device. J. Cell Sci. 113, 3365–3374 (2000).
Zitvogel, L. et al. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell derived exosomes. Nat. Med. 4, 594–600 (1998).
Wilhelm, S. & Hacker, G. Proteolytic specificity of caspases is required to signal the appearance of apoptotic morphology. Eur. J. Cell Biol. 78, 127–133 (1999).
Zocchi, M.R., Poggi, A. & Rubartelli, A. The RGD-containing domain of exogenous HIV-1 Tat inhibits the engulfment of apoptotic bodies by dendritic cells. AIDS 11, 1227–1235 (1997).
Devitt, A. et al. Human CD14 mediates recognition and phagocytosis of apoptotic cells. Nature 392, 505–509 (1998).
Larsson, M., Fonteneau, J.F. & Bhardwaj, N. Dendritic cells resurrect antigens from dead cells. Trends Immunol. 22, 141–148 (2001).
Sieling, P.A. et al. CD1-restricted T cell recognition of microbial lipoglycan antigens. Science 269, 227–230 (1995).
Ochoa, M.T. et al. T-cell release of granulysin contributes to host defense in leprosy. Nat. Med. 7, 174–179 (2001).
Behar, S.M., Porcelli, S.A., Beckman, E.M. & Brenner, M.B. A pathway of costimulation that prevents anergy in CD28− T cells: B7-independent costimulation of CD1-restricted T cells. J. Exp. Med. 182, 2007–2018 (1995).
Jiao, X. et al. Dendritic cells are host cells for mycobacteria in vivo that trigger innate and acquired immunity. J. Immunol. 168, 1294–1301 (2002).
Stenger, S., Niazi, K.R. & Modlin, R.L. Down-regulation of CD1 on antigen-presenting cells by infection with Mycobacterium tuberculosis. J.Immunol. 161, 3582–3588 (1998).
den Haan, J.M.M. & Bevan, M. Antigen presentation to CD8+ T cells: cross-priming in infectious diseases. Curr. Opin. Immunol. 13, 437–441 (2001).
Rodriguez, A., Regnault, A., Kleijmeer, M., Ricciardi-Castagnoli, P. & Amigorena, S. Selective transport of internalized antigens to the cytosol for MHC class I presentation in dendritic cells. Nat. Cell Biol. 1, 362–368 (1999).
Rojas, M., Barrera, L.F., Puzo, G. & Garcia, L.F. Differential induction of apoptosis by virulent Mycobacterium tuberculosis in resistant and susceptible murine macrophages: role of nitric oxide and mycobacterial products. J. Immunol. 159, 1352–1361 (1997).
Klingler, K. et al. Effects of mycobacteria on regulation of apoptosis in mononuclear phagocytes. Infect. Immun. 65, 5272–5278 (1997).
Fayyazi, A. et al. Apoptosis of macrophages and T cells in tuberculosis associated caseous necrosis. J. Pathol. 191, 417–425 (2000).
Yrlid, U. & Wick, M.J. Salmonella-induced apoptosis of infected macrophages results in presentation of a bacteria-encoded antigen after uptake by bystander dendritic cells. J. Exp. Med. 191, 613–624 (2000).
Ronchetti, A. et al. Immunogenicity of apoptotic cells in vivo: role of antigen load, antigen-presenting cells, and cytokines. J. Immunol. 163, 130–136 (1999).
Restifo, N.P. Building better vaccines: how apoptotic cell death can induce inflammation and activate innate and adaptive immunity. Curr. Opin. Immunol. 12, 597–603 (2000).
Acknowledgements
This work was made possible by grants from the Deutsche Forschungsgemeinschaft (SFB421 to S.H.K. and U.E.S.), the Bundesministerium für Bildung und Forschung, a stipend for infectious diseases (U.E.S.) and the EC cluster for tuberculosis vaccine development (S.H.K.). We thank J. Enders and B. Fauler for their excellent technical support; M. Jacobsen and K. Feldmann for peripheral blood lymphocytes derived from tuberculosis patients, and R.S. Jack, M. Sugita, D.B. Young, D.G. Russell, M. Ehlers, J. Belisle and S. Porcelli for kindly sharing reagents with us.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Schaible, U., Winau, F., Sieling, P. et al. Apoptosis facilitates antigen presentation to T lymphocytes through MHC-I and CD1 in tuberculosis. Nat Med 9, 1039–1046 (2003). https://doi.org/10.1038/nm906
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm906
This article is cited by
-
Role of C-terminal domain of Mycobacterium tuberculosis PE6 (Rv0335c) protein in host mitochondrial stress and macrophage apoptosis
Apoptosis (2023)
-
Early alveolar macrophage response and IL-1R-dependent T cell priming determine transmissibility of Mycobacterium tuberculosis strains
Nature Communications (2022)
-
Necroptosis, pyroptosis and apoptosis: an intricate game of cell death
Cellular & Molecular Immunology (2021)
-
Monocyte apoptotic bodies are vehicles for influenza A virus propagation
Communications Biology (2020)
-
Limited recognition of Mycobacterium tuberculosis-infected macrophages by polyclonal CD4 and CD8 T cells from the lungs of infected mice
Mucosal Immunology (2020)