Abstract
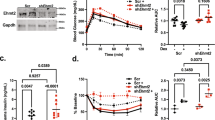
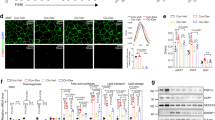
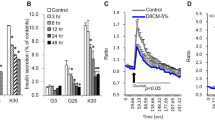
Hypertension and diabetes are common side effects of glucocorticoid treatment. To determine whether peroxisome proliferator–activated receptor-α (PPAR-α) mediates these sequelae, mice deficient in low-density lipoprotein receptor (Ldlr−/−), with (Ppara+/+) or without (Ppara−/−) PPAR-α, were treated chronically with dexamethasone. Ppara+/+, but not Ppara−/−, mice developed hyperglycemia, hyperinsulinemia and hypertension. Similar effects on glucose metabolism were seen in a different model using C57BL/6 mice. Hepatic gluconeogenic gene expression was increased and insulin-mediated suppression of endogenous glucose production was less effective in dexamethasone-treated Ppara+/+ mice. Adenoviral reconstitution of PPAR-α in the livers of nondiabetic, normotensive, dexamethasone-treated Ppara−/− mice induced hyperglycemia, hyperinsulinemia and increased gluconeogenic gene expression. It also increased blood pressure, renin activity, sympathetic nervous activity and renal sodium retention. Human hepatocytes treated with dexamethasone and the PPAR-α agonist Wy14,643 induced PPARA and gluconeogenic gene expression. These results identify hepatic activation of PPAR-α as a mechanism underlying glucocorticoid-induced insulin resistance.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Arner, P., Gunnarsson, R., Blomdahl, S. & Groth, C.G. Some characteristics of steroid diabetes: a study in renal-transplant recipients receiving high-dose corticosteroid therapy. Diabetes 6, 23–25 (1983).
Hricik, D.E., Bartucci, M.R., Moir, E.J., Mayes, J.T. & Schulak, J.A. Effects of steroid withdrawal on posttransplant diabetes mellitus in cyclosporine-treated renal transplant recipients. Transplantation 51, 374–377 (1991).
Williams, L.C. & Nesbitt, L.T. Jr. Update on systemic glucocorticosteroids in dermatology. Dermatol. Clin. 19, 63–77 (2001).
Howlett, T.A., Rees, L.H. & Besser, G.M. Cushing's syndrome. Clin. Endocrinol. Metab. 14, 911–945 (1985).
Rask, E. et al. Tissue-specific dysregulation of cortisol metabolism in human obesity. J. Clin. Endocrinol. Metab. 86, 1418–1421 (2001).
Duclos, M. et al. Fat distribution in obese women is associated with subtle alterations of the hypothalamic-pituitary-adrenal axis activity and sensitivity to glucocorticoids. Clin. Endocrinol. 55, 447–454 (2001).
Levitt, N.S. et al. Impaired glucose tolerance and elevated blood pressure in low birth weight, nonobese, young South African adults: early programming of cortisol axis. J. Clin. Endocrinol. Metab. 85, 4611–4618 (2000).
Andrew, R., Gale, C.R., Walker, B.R., Seckl, J.R. & Martyn, C.N. Glucocorticoid metabolism and the metabolic syndrome: associations in an elderly cohort. Exp. Clin. Endocrinol. Diabetes 110, 284–290 (2002).
Tauchmanova, T. et al. Patients with subclinical Cushing's syndrome due to adrenal adenoma have increased cardiovascular risk. J. Clin. Endocrinol. Metab. 87, 4872–4878 (2002).
Masuzaki, H. et al. A transgenic model of visceral obesity and the metabolic syndrome. Science 294, 2166–2170 (2001).
Ginsberg, H.N. Insulin resistance and cardiovascular disease. J. Clin. Invest. 106, 453–458 (2000).
Djurhuus, C.B. et al. Effects of cortisol on lipolysis and regional interstitial glycerol levels in humans. Am. J. Physiol. 283, E172–E177 (2002).
Ekstrand, A., Saloranta, C., Ahonen, J., Gronhagen-Riska, C. & Groop, L.C. Reversal of steroid-induced insulin resistance by a nicotinic-acid derivative in man. Metabolism 41, 692–697 (1992).
Berger, J. & Moller, D.E. The mechanisms of action of PPARs. Annu. Rev. Med. 53, 409–435 (2002).
Lemberger, T. et al. Regulation of the peroxisome proliferator-activated receptor alpha gene by glucocorticoids. J. Biol. Chem. 269, 24527–24530 (1994).
Lemberger, T. et al. Expression of the peroxisome proliferator-activated receptor alpha gene is stimulated by stress and follows a diurnal rhythm. J. Biol. Chem. 271, 1764–1769 (1996).
Tordjman, K. et al. PPARα deficiency reduces insulin resistance and atherosclerosis in apoE-null mice. J. Clin. Invest. 107, 1025–1034 (2001).
Guerre-Millo, M. et al. PPAR-alpha-null mice are protected from high-fat diet-induced insulin resistance. Diabetes 50, 2809–2814 (2001).
Towler, D.A., Bidder, M., Latifi, T., Coleman, T. & Semenkovich, C.F. Diet-induced diabetes activates an osteogenic gene regulatory program in the aortas of low density lipoprotein receptor-deficient mice. J. Biol. Chem. 273, 30427–30434 (1998).
Schreyer, S.A., Vick, C., Lystig, T.C., Mystkowski, P. & LeBoeuf, R.C. LDL receptor but not apolipoprotein E deficiency increases diet-induced obesity and diabetes in mice. Am. J. Physiol. 282, E207–E214 (2002).
Tordjman, K. et al. PPARalpha suppresses insulin secretion and induces UCP2 in insulinoma cells. J. Lipid Res. 43, 936–943 (2002).
Yoon, J.C. et al. Control of hepatic gluconeogenesis through the transcriptional coactivator PGC-1. Nature 413, 131–138 (2001).
Herzig, S. et al. CREB regulates hepatic gluconeogenesis through the coactivator PGC-1. Nature 413, 179–183 (2001).
Peters, J.M. et al. Alterations in lipoprotein metabolism in peroxisome proliferator-activated receptor α-deficient mice. J. Biol. Chem. 43, 27307–27312 (1997).
Dirlewanger, M. et al. Effects of glucocorticoids on hepatic sensitivity to insulin and glucagon in man. Clin. Nutr. 19, 29–34 (2000).
Tappy, L. et al. Mechanisms of dexamethasone-induced insulin resistance in healthy humans. J. Clin. Endocrinol. Metab. 79, 1063–1069 (1994).
Goodpaster, B.H., Kelley, D.E., Wing, R.R., Meier, A. & Thaete, F.L. Effects of weight loss on regional fat distribution and insulin sensitivity in obesity. Diabetes 48, 839–847 (1999).
Khani, S. & Tayek, J.A. Cortisol increases gluconeogenesis in humans: its role in the metabolic syndrome. Clin. Sci. 101, 739–747 (2001).
Lawrence, J.W. et al. Differential gene regulation in human versus rodent hepatocytes by peroxisome proliferator-activated receptor (PPAR) alpha. PPAR alpha fails to induce peroxisome proliferation-associated genes in human cells independently of the level of receptor expression. J. Biol. Chem. 276, 31521–31527 (2001).
Vidal-Puig, A. et al. Regulation of PPARγ gene expression by nutrition and obesity in rodents. J. Clin. Invest. 97, 2553–2561 (1996).
Matsusue, K. et al. Liver-specific disruption of PPARgamma in leptin-deficient mice improves fatty liver but aggravates diabetic phenotypes. J. Clin. Invest. 111, 737–747 (2003).
Hermanowski-Vosatka, A. et al. PPARα agonists reduce 11β-hydroxysteroid dehydrogenase type 1 in the liver. Biochem. Biophys. Res. Comm. 279, 330–336 (2000).
Kotelevtsev, Y. et al. 11beta-hydroxysteroid dehydrogenase type 1 knockout mice show attenuated glucocorticoid-inducible responses and resist hyperglycemia on obesity or stress. Proc. Natl. Acad. Sci. USA 94, 14924–14929 (1997).
Kopf, D. et al. Insulin sensitivity and sodium excretion in normotensive offspring and hypertensive patients. Metabolism 50, 929–935 (2001).
Orbach, J. & Andrews, W.H. Stimulation of afferent nerve terminals in the perfused rabbit liver by sodium salts of some long-chain fatty acids. Q. J. Exp. Physiol. Cogn. Med. Sci. 58, 267–274 (1973).
Randich, A., Spraggins, D.S., Cox, J.E., Meller, S.T. & Kelm, G.R. Jejunal or portal vein infusions of lipids increase hepatic vagal afferent activity. NeuroReport 12, 3101–3105 (2001).
Vacca, G., Chiorboli, E., Grossini, E. & Papillo, B. The effect of distension of the stomach on plasma renin activity in the anesthetized pig. Cardioscience 5, 261–267 (1994).
Grekin, R.J., Vollmer, A.P. & Sider, R.S. Pressor effects of portal venous oleate infusion. A proposed mechanism for obesity hypertension. Hypertension 26, 193–198 (1995).
Benthem, L. et al. Excess portal venous long-chain fatty acids induce syndrome X via HPA axis and sympathetic activation. Am. J. Physiol. 279, E1286–E1293 (2000).
Marshall, B.A. et al. Relative hypoglycemia and hyperinsulinemia in mice with heterozygous lipoprotein lipase (LPL) deficiency. Islet LPL regulates insulin secretion. J. Biol. Chem. 274, 27426–27432 (1999).
Li, B. et al. Skeletal muscle respiratory uncoupling prevents diet-induced obesity and insulin resistance in mice. Nat. Med. 6, 1115–1120 (2000).
Bernal-Mizrachi, C. et al. Respiratory uncoupling lowers blood pressure through a leptin-dependent mechanism in genetically obese mice. Arterioscler. Thromb. Vasc. Biol. 22, 961–968 (2002).
Marshall, B.A., Hansen, P.A., Ensor, N.J., Ogden, M.A. & Mueckler, M. GLUT-1 or GLUT-4 transgenes in obese mice improve glucose tolerance but do not prevent insulin resistance. Am. J. Physiol. 276, E390–E400 (1999).
Hawkins, M. et al. Discordant effects of glucosamine on insulin-stimulated glucose metabolism and phosphatidylinositol 3-kinase activity. J. Biol. Chem. 274, 31312–31319 (1999).
Lehman, J.J. et al. Peroxisome proliferator-activated receptor (coactivator-1) promotes cardiac mitochondrial biogenesis. J. Clin. Invest. 106, 847–856 (2000).
Heximer, S.P. et al. Hypertension and prolonged vasoconstrictor signaling in RGS2-deficient mice. J. Clin. Invest. 111, 445–452 (2003).
Acknowledgements
This study was supported by National Institutes of Health grants HL58427 and AG20091, Clinical Nutrition Research Unit grant DK56341 and Diabetes Research and Training Center grant DK20579. C.B.-M. was supported by an American Diabetes Association–Johnson & Johnson Mentor-Based Postdoctoral Fellowship. We thank E. Bernal-Mizrachi and K.S. Polonsky for suggestions and support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
D.P.K. is a scientific consultant for Merck Research Labs and gives talks supported by Merck. This work was not supported by or related to this affiliation.
Supplementary information
Rights and permissions
About this article
Cite this article
Bernal-Mizrachi, C., Weng, S., Feng, C. et al. Dexamethasone induction of hypertension and diabetes is PPAR-α dependent in LDL receptor–null mice. Nat Med 9, 1069–1075 (2003). https://doi.org/10.1038/nm898
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm898
This article is cited by
-
Embryonic vitamin D deficiency programs hematopoietic stem cells to induce type 2 diabetes
Nature Communications (2023)
-
Interactions between nuclear receptors glucocorticoid receptor α and peroxisome proliferator–activated receptor α form a negative feedback loop
Reviews in Endocrine and Metabolic Disorders (2022)
-
FGF21 Enhances Therapeutic Efficacy and Reduces Side Effects of Dexamethasone in Treatment of Rheumatoid Arthritis
Inflammation (2021)
-
Macrophage secretion of miR-106b-5p causes renin-dependent hypertension
Nature Communications (2020)
-
Metabolic aspects in NAFLD, NASH and hepatocellular carcinoma: the role of PGC1 coactivators
Nature Reviews Gastroenterology & Hepatology (2019)