Abstract
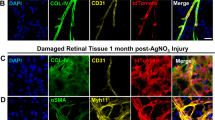
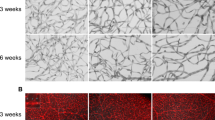
Adult bone marrow (BM) contains cells capable of differentiating along hematopoietic (Lin+) or non-hematopoietic (Lin−) lineages. Lin− hematopoietic stem cells (HSCs) have recently been shown to contain a population of endothelial precursor cells (EPCs) capable of forming blood vessels. Here we show that intravitreally injected Lin− BM cells selectively target retinal astrocytes, cells that serve as a template for both developmental and injury-associated retinal angiogenesis. When Lin− BM cells were injected into neonatal mouse eyes, they extensively and stably incorporated into forming retinal vasculature. When EPC-enriched HSCs were injected into the eyes of neonatal rd/rd mice, whose vasculature ordinarily degenerates with age, they rescued and maintained a normal vasculature. In contrast, normal retinal angiogenesis was inhibited when EPCs expressing a potent angiostatic protein were injected. We have demonstrated that Lin− BM cells and astrocytes specifically interact with one another during normal angiogenesis and pathological vascular degeneration in the retina. Selective targeting with Lin− HSC may be a useful therapeutic approach for the treatment of many ocular diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Asahara, T. et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science 275, 964–967 (1997).
Lyden, D. et al. Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nature Med. 7, 1194–1201 (2001).
Kalka, C. et al. Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc. Natl. Acad. Sci. USA 97, 3422–3427 (2000).
Kocher, A.A. et al. Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeLing and improves cardiac function. Nature Med. 7, 430–436 (2001).
Lagasse, E. et al. Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nature Med. 6, 1229–1234 (2000).
Priller, J. et al. Targeting gene-modified hematopoietic cells to the central nervous system: use of green fluorescent protein uncovers microglial engraftment. Nature Med. 7, 1356–1361 (2001).
Orlic, D. et al. Mobilized bone marrow cells repair the infarcted heart, improving function and survival. Proc. Natl. Acad. Sci. USA 98, 10344–10349 (2001).
Zhang, Y. & Stone, J. Role of astrocytes in the control of developing retinal vessels. Invest. Ophthalmol. Vis. Sci. 38, 1653–1666 (1997).
Dyer, M.A. & Cepko, C.L. Control of Muller glial cell proliferation and activation following retinal injury. Nature Neurosci. 3, 873–880 (2000).
MacLaren, R.E. Development and role of retinal glia in regeneration of ganglion cells following retinal injury. Br. J. Ophthalmol. 80, 458–464 (1996).
Nork, T.M., Wallow, I.H., Sramek, S.J. & Anderson, G. Muller's cell involvement in proliferative diabetic retinopathy. Arch. Ophthalmol. 105, 1424–1429 (1987).
Ohira, A. & de Juan, E. Jr Characterization of glial involvement in proliferative diabetic retinopathy. Ophthalmologica 201, 187–195 (1990).
Amin, R.H. et al. Vascular endothelial growth factor is present in glial cells of the retina and optic nerve of human subjects with nonproliferative diabetic retinopathy. Invest. Ophthalmol. Vis. Sci. 38, 36–47 (1997).
McLeod, D.S., D'Anna, S.A. & Lutty, G.A. Clinical and histopathologic features of canine oxygen-induced proliferative retinopathy. Invest. Ophthalmol. Vis. Sci. 39, 1918–1932 (1998).
Ridet, J.L., Malhotra, S.K., Privat, A. & Gage, F.H. Reactive astrocytes: cellular and molecular cues to biological function. Trends Neurosci. 20, 570–577 (1997).
Schnitzer, J. Astrocytes in the guinea pig, horse, and monkey retina: Their occurrence coincides with the presence of blood vessels. Glia 1, 74–89 (1988).
Wakasugi, K. et al. A human aminoacyl-tRNA synthetase as a regulator of angiogenesis. Proc. Natl. Acad. Sci. USA 99, 173–177 (2002).
Otani, A. et al. A fragment of human TrpRS as a potent antagonist of ocular angiogenesis. Proc. Natl. Acad. Sci. USA 99, 178–183 (2002).
Madigan, M.C., Penfold, P.L., Provis, J.M., BaLind, T.K. & Billson, F.A. Intermediate filament expression in human retinal macroglia. Histopathologic changes associated with age-related macular degeneration. Retina 14, 65–74 (1994).
Mizutani, M., Gerhardinger, C. & Lorenzi, M. Muller cell changes in human diabetic retinopathy. Diabetes 47, 445–449 (1998).
Rungger-Brandle, E., Dosso, A.A. & Leuenberger, P.M. Glial reactivity, an early feature of diabetic retinopathy. Invest. Ophthalmol. Vis. Sci. 41, 1971–1980 (2000).
Davidoff, A.M. et al. Bone marrow-derived cells contribute to tumor neovasculature and, when modified to express an angiogenesis inhibitor, can restrict tumor growth in mice. CLin. Cancer Res. 7, 2870–2879 (2001).
Wang, S., Villegas-Perez, M.P., Vidal-Sanz, M. & Lund, R.D. Progressive optic axon dystrophy and vacuslar changes in rd mice. Invest. Ophthalmol. Vis. Sci. 41, 537–545 (2000).
Matthes, M.T. & Bok, D. Blood vascular abnormalities in the degenerative mouse retina (C57BL/6J-rd le). Invest. Ophthalmol. Vis. Sci. 25, 364–369 (1984).
Grant, M.B. et al. Adult hematopoietic stem cells provide functional hemangioblast activity during retinal neovascularization. Nature Med. 8, 607–612 (2002).
Carmeliet, P. VEGF gene therapy: stimulating angiogenesis or angioma-genesis? Nature Med. 6, 1102–1123 (2000).
Ahmad, I., Tang, L. & Pham, H. Identification of neural progenitors in the adult mammalian eye. Biochem. Biophys. Res. Commun. 270, 517–521 (2000).
Tropepe, V. et al. Retinal stem cells in the adult mammalian eye. Science 287, 2032–2036 (2000).
Acknowledgements
We thank the TSRI FACS Facility; E. Aguilar and R. Gariano for assistance with the laser-induced retinal injury model; S. Fallon, M. Ritter, M. Dorrell and G. Nemerow for helpful suggestions and J. Isner for insightful discussions. This work was supported by the National Eye Institute (EY11254 and EY12599 to M.F.), a Core Grant for Vision Research (EY 12598), the National Cancer Institute (CA92577 to P.S.), the Robert Mealey Program for the Study of Macular Degenerations (to M.F.), and the National Foundation for Cancer Research (P.S.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Otani, A., Kinder, K., Ewalt, K. et al. Bone marrow–derived stem cells target retinal astrocytes and can promote or inhibit retinal angiogenesis. Nat Med 8, 1004–1010 (2002). https://doi.org/10.1038/nm744
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm744
This article is cited by
-
Purification and characterization of human adipose-resident microvascular endothelial progenitor cells
Scientific Reports (2022)
-
Lipids, hyperreflective crystalline deposits and diabetic retinopathy: potential systemic and retinal-specific effect of lipid-lowering therapies
Diabetologia (2022)
-
Current understanding of the molecular and cellular pathology of diabetic retinopathy
Nature Reviews Endocrinology (2021)
-
Retinopathy of prematurity: inflammation, choroidal degeneration, and novel promising therapeutic strategies
Journal of Neuroinflammation (2017)
-
In Situ Pluripotency Factor Expression Promotes Functional Recovery From Cerebral Ischemia
Molecular Therapy (2016)