Abstract
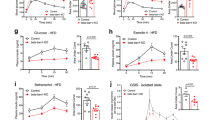
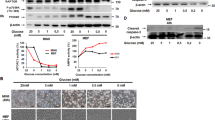
The proapoptotic BCL-2 family member BAD resides in a glucokinase-containing complex that regulates glucose-driven mitochondrial respiration. Here, we present genetic evidence of a physiologic role for BAD in glucose-stimulated insulin secretion by beta cells. This novel function of BAD is specifically dependent upon the phosphorylation of its BH3 sequence, previously defined as an essential death domain. We highlight the pharmacologic relevance of phosphorylated BAD BH3 by using cell-permeable, hydrocarbon-stapled BAD BH3 helices that target glucokinase, restore glucose-driven mitochondrial respiration and correct the insulin secretory response in Bad-deficient islets. Our studies uncover an alternative target and function for the BAD BH3 domain and emphasize the therapeutic potential of phosphorylated BAD BH3 mimetics in selectively restoring beta cell function. Furthermore, we show that BAD regulates the physiologic adaptation of beta cell mass during high-fat feeding. Our findings provide genetic proof of the bifunctional activities of BAD in both beta cell survival and insulin secretion.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Green, D.R. & Kroemer, G. The pathophysiology of mitochondrial cell death. Science 305, 626–629 (2004).
Danial, N.N. et al. BAD and glucokinase reside in a mitochondrial complex that integrates glycolysis and apoptosis. Nature 424, 952–956 (2003).
Plas, D.R. & Thompson, C.B. Cell metabolism in the regulation of programmed cell death. Trends Endocrinol. Metab. 13, 75–78 (2002).
Pinton, P. & Rizzuto, R. Bcl-2 and Ca2+ homeostasis in the endoplasmic reticulum. Cell Death Differ. 13, 1409–1418 (2006).
Karbowski, M., Norris, K.L., Cleland, M.M., Jeong, S.Y. & Youle, R.J. Role of Bax and Bak in mitochondrial morphogenesis. Nature 443, 658–662 (2006).
Harada, H. et al. Phosphorylation and inactivation of BAD by mitochondria-anchored protein kinase A. Mol. Cell 3, 413–422 (1999).
Harada, H., Andersen, J.S., Mann, M., Terada, N. & Korsmeyer, S.J. p70S6 kinase signals cell survival as well as growth, inactivating the pro-apoptotic molecule BAD. Proc. Natl. Acad. Sci. USA 98, 9666–9670 (2001).
Magnuson, M.A. & Matschinsky, F.M. Glucokinase and Glycemic Disease: From Basics to Novel Therapeutics. 1–17, 42–64, 360–397 (Karger, Basel, Switzerland, 2004).
Bali, D. et al. Animal model for maturity-onset diabetes of the young generated by disruption of the mouse glucokinase gene. J. Biol. Chem. 270, 21464–21467 (1995).
Grupe, A. et al. Transgenic knockouts reveal a critical requirement for pancreatic beta cell glucokinase in maintaining glucose homeostasis. Cell 83, 69–78 (1995).
Postic, C. et al. Dual roles for glucokinase in glucose homeostasis as determined by liver and pancreatic beta cell–specific gene knock-outs using Cre recombinase. J. Biol. Chem. 274, 305–315 (1999).
Terauchi, Y. et al. Pancreatic beta cell–specific targeted disruption of glucokinase gene. Diabetes mellitus due to defective insulin secretion to glucose. J. Biol. Chem. 270, 30253–30256 (1995).
Wiederkehr, A. & Wollheim, C.B. Minireview: implication of mitochondria in insulin secretion and action. Endocrinology 147, 2643–2649 (2006).
Newgard, C.B. & McGarry, J.D. Metabolic coupling factors in pancreatic beta cell signal transduction. Annu. Rev. Biochem. 64, 689–719 (1995).
Berggren, P.O. & Larsson, O. Ca2+ and pancreatic B-cell function. Biochem. Soc. Trans. 22, 12–18 (1994).
Gao, Z. et al. Distinguishing features of leucine and α-ketoisocaproate sensing in pancreatic beta cells. Endocrinology 144, 1949–1957 (2003).
Proks, P., Reimann, F., Green, N., Gribble, F. & Ashcroft, F. Sulfonylurea stimulation of insulin secretion. Diabetes 51 Suppl 3, S368–S376 (2002).
Arden, C., Baltrusch, S. & Agius, L. Glucokinase regulatory protein is associated with mitochondria in hepatocytes. FEBS Lett. 580, 2065–2070 (2006).
Antinozzi, P.A., Ishihara, H., Newgard, C.B. & Wollheim, C.B. Mitochondrial metabolism sets the maximal limit of fuel-stimulated insulin secretion in a model pancreatic beta cell: a survey of four fuel secretagogues. J. Biol. Chem. 277, 11746–11755 (2002).
Liang, Y. et al. Glucose metabolism and insulin release in mouse beta HC9 cells, as model for wild-type pancreatic beta cells. Am. J. Physiol. 270, E846–E857 (1996).
Heart, E., Corkey, R.F., Wikstrom, J.D., Shirihai, O.S. & Corkey, B.E. Glucose-dependent increase in mitochondrial membrane potential, but not cytoplasmic calcium, correlates with insulin secretion in single islet cells. Am. J. Physiol. Endocrinol. Metab. 290, E143–E148 (2006).
Zha, J. et al. BH3 domain of BAD is required for heterodimerization with BCL-XL and pro-apoptotic activity. J. Biol. Chem. 272, 24101–24104 (1997).
Datta, S.R. et al. Survival factor–mediated BAD phosphorylation raises the mitochondrial threshold for apoptosis. Dev. Cell 3, 631–643 (2002).
Zha, J., Harada, H., Yang, E., Jockel, J. & Korsmeyer, S.J. Serine phosphorylation of death agonist BAD in response to survival factor results in binding to 14–3-3 not BCL-XL . Cell 87, 619–628 (1996).
Walensky, L.D. et al. Activation of apoptosis in vivo by a hydrocarbon-stapled BH3 helix. Science 305, 1466–1470 (2004).
Walensky, L.D. et al. A stapled BID BH3 helix directly binds and activates BAX. Mol. Cell 24, 199–210 (2006).
Datta, S.R. et al. 14–3-3 proteins and survival kinases cooperate to inactivate BAD by BH3 domain phosphorylation. Mol. Cell 6, 41–51 (2000).
Burch, P.T. et al. Adaptation of glycolytic enzymes: glucose use and insulin release in rat pancreatic islets during fasting and refeeding. Diabetes 30, 923–928 (1981).
Saghatelian, A., Jessani, N., Joseph, A., Humphrey, M. & Cravatt, B.F. Activity-based probes for the proteomic profiling of metalloproteases. Proc. Natl. Acad. Sci. USA 101, 10000–10005 (2004).
Accili, D., Kido, Y., Nakae, J., Lauro, D. & Park, B.C. Genetics of type 2 diabetes: insight from targeted mouse mutants. Curr. Mol. Med. 1, 9–23 (2001).
Bell, G.I. & Polonsky, K.S. Diabetes mellitus and genetically programmed defects in beta cell function. Nature 414, 788–791 (2001).
Dickson, L.M. & Rhodes, C.J. Pancreatic beta cell growth and survival in the onset of type 2 diabetes: a role for protein kinase B in the Akt? Am. J. Physiol. Endocrinol. Metab. 287, E192–E198 (2004).
Weir, G.C., Laybutt, D.R., Kaneto, H., Bonner-Weir, S. & Sharma, A. Beta cell adaptation and decompensation during the progression of diabetes. Diabetes 50 Suppl 1, S154–S159 (2001).
Prentki, M., Joly, E., El-Assaad, W. & Roduit, R. Malonyl-CoA signaling, lipid partitioning, and glucolipotoxicity: role in beta cell adaptation and failure in the etiology of diabetes. Diabetes 51 Suppl 3, S405–S413 (2002).
Jetton, T.L. et al. Mechanisms of compensatory beta cell growth in insulin-resistant rats: roles of Akt kinase. Diabetes 54, 2294–2304 (2005).
Zhou, Y.P. et al. Overexpression of Bcl-XL in beta cells prevents cell death but impairs mitochondrial signal for insulin secretion. Am. J. Physiol. Endocrinol. Metab. 278, E340–E351 (2000).
Patrucco, E. et al. PI3K-γ modulates the cardiac response to chronic pressure overload by distinct kinase-dependent and -independent effects. Cell 118, 375–387 (2004).
Hara, M.R. & Snyder, S.H. Nitric oxide-GAPDH-Siah: a novel cell death cascade. Cell. Mol. Neurobiol. 26, 525–536 (2006).
Tan, Y., Demeter, M.R., Ruan, H. & Comb, M.J. BAD Ser-155 phosphorylation regulates BAD/Bcl-XL interaction and cell survival. J. Biol. Chem. 275, 25865–25869 (2000).
Baggio, L.L. & Drucker, D.J. Biology of incretins: GLP-1 and GIP. Gastroenterology 132, 2131–2157 (2007).
Jhala, U.S. et al. cAMP promotes pancreatic beta cell survival via CREB-mediated induction of IRS2. Genes Dev. 17, 1575–1580 (2003).
Pende, M. et al. Hypoinsulinaemia, glucose intolerance and diminished beta-cell size in S6K1-deficient mice. Nature 408, 994–997 (2000).
Tuttle, R.L. et al. Regulation of pancreatic beta cell growth and survival by the serine/threonine protein kinase Akt1/PKBα. Nat. Med. 7, 1133–1137 (2001).
Ranger, A.M. et al. Bad-deficient mice develop diffuse large B cell lymphoma. Proc. Natl. Acad. Sci. USA 100, 9324–9329 (2003).
Winzell, M.S. & Ahren, B. The high-fat diet–fed mouse: a model for studying mechanisms and treatment of impaired glucose tolerance and type 2 diabetes. Diabetes 53 Suppl 3, S215–S219 (2004).
Larsson, O., Deeney, J.T., Branstrom, R., Berggren, P.O. & Corkey, B.E. Activation of the ATP-sensitive K+ channel by long chain acyl-CoA. A role in modulation of pancreatic beta cell glucose sensitivity. J. Biol. Chem. 271, 10623–10626 (1996).
Schultz, V., Sussman, I., Bokvist, K. & Tornheim, K. Bioluminometric assay of ADP and ATP at high ATP/ADP ratios: assay of ADP after enzymatic removal of ATP. Anal. Biochem. 215, 302–304 (1993).
Trus, M.D. et al. Regulation of glucose metabolism in pancreatic islets. Diabetes 30, 911–922 (1981).
He, T.C. et al. A simplified system for generating recombinant adenoviruses. Proc. Natl. Acad. Sci. USA 95, 2509–2514 (1998).
Zhou, Y.P. et al. Overexpression of repressive cAMP response element modulators in high glucose and fatty acid-treated rat islets. A common mechanism for glucose toxicity and lipotoxicity? J. Biol. Chem. 278, 51316–51323 (2003).
Acknowledgements
We thank M. Godes and C. Gramm for technical assistance, R. Pasquier and B. Szlyk for islet isolation and animal husbandry, G. Weir (Joslin Diabetes Center, Boston, MA) for antibody to glucokinase, B. Spiegelman, B. Malynn, M. Vander Heiden, A. Schinzel, J. Labelle, G. Verdine, F. Bernal, A. Saghatelian, A.-M. Richards and G. Yaney for helpful discussion and E. Smith for manuscript preparation. N.N.D. and L.D.W. are recipients of the Burroughs Wellcome Fund Career Award in Biomedical Sciences. This work was supported by US National Institute of Health grants K01CA10659 (N.N.D.), 5R01CA50239 and 5R01DK68781 (S.J.K.), 5K08HL074049 (L.D.W.) and by Charles E. Culpeper Scholarship in Medical Science (L.D.W.).
Author information
Authors and Affiliations
Contributions
N.N.D. planned and performed the in vivo and in vitro experiments, supervised the project and wrote the manuscript. J.K.F. and K.R. assisted with conducting experiments. L.D.W., K.L.P., G.H.B. and J.M. designed and synthesized the SAHB compounds and characterized their chemical and biophysical properties. C.-Y.Z. made conceptual contributions and helped with data interpretation. C.S.C. performed the hyperglycemic clamp studies with help from A.K., S.N., S.K. and supervision from G.I.S. A.J.A.M. and J.D.W. performed the measurements of mitochondrial membrane potential with supervision from O.S.S. S.R.D. generated the BadS155A knock-in model with supervision from M.E.G. J.T.D. helped with islet perifusion studies. B.E.C. provided critical guidance and expertise. B.B.L. provided advice. The late S.J.K. provided the mentorship and laboratory resources that catalyzed the initial studies.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figs. 1–4, Supplementary Table 1 and Supplementary Methods (PDF 645 kb)
Rights and permissions
About this article
Cite this article
Danial, N., Walensky, L., Zhang, CY. et al. Dual role of proapoptotic BAD in insulin secretion and beta cell survival. Nat Med 14, 144–153 (2008). https://doi.org/10.1038/nm1717
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm1717
This article is cited by
-
A stapled lipopeptide platform for preventing and treating highly pathogenic viruses of pandemic potential
Nature Communications (2024)
-
Oxidative stress in obesity-associated hepatocellular carcinoma: sources, signaling and therapeutic challenges
Oncogene (2021)
-
BAD regulates mammary gland morphogenesis by 4E-BP1-mediated control of localized translation in mouse and human models
Nature Communications (2021)
-
A BAD portion of glucose can be good for inflamed beta cells
Nature Metabolism (2020)
-
Bax and Bak jointly control survival and dampen the early unfolded protein response in pancreatic β-cells under glucolipotoxic stress
Scientific Reports (2020)