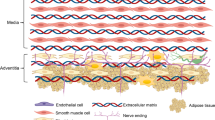
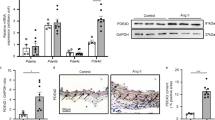
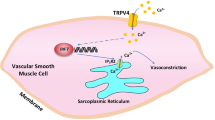
Abstract
The tone of vascular smooth muscle cells is a primary determinant of the total peripheral vascular resistance and hence the arterial blood pressure. Most forms of hypertension ultimately result from an increased vascular tone that leads to an elevated total peripheral resistance1,2,3. Regulation of vascular resistance under normotensive and hypertensive conditions involves multiple mediators, many of which act through G protein–coupled receptors on vascular smooth muscle cells4. Receptors that mediate vasoconstriction couple with the G-proteins Gq-G11 and G12-G13 to stimulate phosphorylation of myosin light chain (MLC) via the Ca2+/MLC kinase– and Rho/Rho kinase–mediated signaling pathways, respectively4,5,6. Using genetically altered mouse models that allow for the acute abrogation of both signaling pathways by inducible Cre/loxP-mediated mutagenesis in smooth muscle cells, we show that Gq-G11–mediated signaling in smooth muscle cells is required for maintenance of basal blood pressure and for the development of salt-induced hypertension. In contrast, lack of G12-G13, as well as of their major effector, the leukemia-associated Rho guanine nucleotide exchange factor (LARG), did not alter normal blood pressure regulation but did block the development of salt-induced hypertension. This identifies the G12-G13–LARG–mediated signaling pathway as a new target for antihypertensive therapies that would be expected to leave normal blood pressure regulation unaffected.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
09 January 2008
In the version of this article initially published, the name of one author was incorrectly listed as Silvio Gutkind. The correct name is J. Silvio Gutkind. The error has been corrected in the HTML and PDF versions of the article.
References
Brown, R., Mullins, J. & Webb, D.J. Mechanisms and molecular pathways in hypertension. in Molecular Basis of Cardiovascular Disease (ed. Chien, K.R.) 566–647 (Saunders, Philadelphia, 2004).
Cowley, A.W., Jr. Long-term control of arterial blood pressure. Physiol. Rev. 72, 231–300 (1992).
Lifton, R.P., Gharavi, A.G. & Geller, D.S. Molecular mechanisms of human hypertension. Cell 104, 545–556 (2001).
Maguire, J.J. & Davenport, A.P. Regulation of vascular reactivity by established and emerging GPCRs. Trends Pharmacol. Sci. 26, 448–454 (2005).
Gohla, A., Schultz, G. & Offermanns, S. Role for G12/G13 in agonist-induced vascular smooth muscle cell contraction. Circ. Res. 87, 221–227 (2000).
Somlyo, A.P. & Somlyo, A.V. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol. Rev. 83, 1325–1358 (2003).
Chobanian, A.V. et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 42, 1206–1252 (2003).
Ezzati, M. et al. Rethinking the “diseases of affluence” paradigm: global patterns of nutritional risks in relation to economic development. PLoS Med. 2, e133 (2005).
O'Shaughnessy, K.M. & Karet, F.E. Salt handling and hypertension. J. Clin. Invest. 113, 1075–1081 (2004).
Meneton, P., Jeunemaitre, X., de Wardener, H.E. & MacGregor, G.A. Links between dietary salt intake, renal salt handling, blood pressure and cardiovascular diseases. Physiol. Rev. 85, 679–715 (2005).
Iwamoto, T. et al. Salt-sensitive hypertension is triggered by Ca2+ entry via Na+/Ca2+ exchanger type-1 in vascular smooth muscle. Nat. Med. 10, 1193–1199 (2004).
Dostanic, I. et al. The alpha2-isoform of Na-K-ATPase mediates ouabain-induced hypertension in mice and increased vascular contractility in vitro. Am. J. Physiol. Heart Circ. Physiol. 288, H477–H485 (2005).
Blaustein, M.P., Zhang, J., Chen, L. & Hamilton, B.P. How does salt retention raise blood pressure? Am. J. Physiol. Regul. Integr. Comp. Physiol. 290, R514–R523 (2006).
Narumiya, S., Ishizaki, T. & Watanabe, N. Rho effectors and reorganization of actin cytoskeleton. FEBS Lett. 410, 68–72 (1997).
Fukata, Y., Amano, M. & Kaibuchi, K. Rho-Rho-kinase pathway in smooth muscle contraction and cytoskeletal reorganization of non-muscle cells. Trends Pharmacol. Sci. 22, 32–39 (2001).
Fukuhara, S., Chikumi, H. & Gutkind, J.S. RGS-containing RhoGEFs: the missing link between transforming G proteins and Rho? Oncogene 20, 1661–1668 (2001).
Wettschureck, N. et al. Absence of pressure overload induced myocardial hypertrophy after conditional inactivation of Gαq/Gα11 in cardiomyocytes. Nat. Med. 7, 1236–1240 (2001).
Moers, A. et al. G13 is an essential mediator of platelet activation in hemostasis and thrombosis. Nat. Med. 9, 1418–1422 (2003).
Indra, A.K. et al. Temporally-controlled site-specific mutagenesis in the basal layer of the epidermis: comparison of the recombinase activity of the tamoxifen-inducible Cre-ERT and Cre-ERT2 recombinases. Nucleic Acids Res. 27, 4324–4327 (1999).
Becknell, B. et al. Characterization of leukemia-associated Rho guanine nucleotide exchange factor (LARG) expression during murine development. Cell Tissue Res. 314, 361–366 (2003).
Moriki, N. et al. RhoA activation in vascular smooth muscle cells from stroke-prone spontaneously hypertensive rats. Hypertens. Res. 27, 263–270 (2004).
Heximer, S.P. et al. Hypertension and prolonged vasoconstrictor signaling in RGS2-deficient mice. J. Clin. Invest. 111, 445–452 (2003).
Tang, K.M. et al. Regulator of G-protein signaling-2 mediates vascular smooth muscle relaxation and blood pressure. Nat. Med. 9, 1506–1512 (2003).
Seasholtz, T.M. & Brown, J.H. Rho signaling in vascular diseases. Mol. Interv. 4, 348–357 (2004).
Seko, T. et al. Activation of RhoA and inhibition of myosin phosphatase as important components in hypertension in vascular smooth muscle. Circ. Res. 92, 411–418 (2003).
Uehata, M. et al. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature 389, 990–994 (1997).
Amano, M., Fukata, Y. & Kaibuchi, K. Regulation and functions of Rho-associated kinase. Exp. Cell Res. 261, 44–51 (2000).
Noma, K., Oyama, N. & Liao, J.K. Physiological role of ROCKs in the cardiovascular system. Am. J. Physiol. Cell Physiol. 290, C661–C668 (2006).
Khalil, R.A. Dietary salt and hypertension: new molecular targets add more spice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 290, R509–R513 (2006).
Mills, P.A. et al. A new method for measurement of blood pressure, heart rate, and activity in the mouse by radiotelemetry. J. Appl. Physiol. 88, 1537–1544 (2000).
Acknowledgements
The authors are grateful to M. Ritzal and T. Németh for their expert technical assistance. Z.B. and B.H. were supported by a Marie Curie Individual Fellowship and a European Molecular Biology Organization Fellowship, respectively. This work was supported by the Deutsche Forschungsgemeinschaft (Of 19/9) and the Fonds der Chemischen Industrie.
Author information
Authors and Affiliations
Contributions
A.W. planned and performed most in vivo experiments, analyzed SMMHC-CreERT2 mice and was involved in writing the manuscript, Z.B. and M.L. planned and performed most in vitro experiments, B.L. helped generate and analyze SMMHC-CreERT2 mice, N.W. and S.G. helped perform in vivo experiments, P.Ö. and B.H. helped perform in vitro experiments, C.M.-G. performed DOCA level determinations, E.G. helped generate SMMHC-CreERT2 mice, B.L. helped perform in vivo experiments, G.S. helped generate SMMHC-CreERT2 mice, S.G. generated LARG-deficient mice, and S.O. planned and supervised the project and wrote the manuscript.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figs. 1–3 and Supplementary Methods (PDF 299 kb)
Rights and permissions
About this article
Cite this article
Wirth, A., Benyó, Z., Lukasova, M. et al. G12-G13–LARG–mediated signaling in vascular smooth muscle is required for salt-induced hypertension. Nat Med 14, 64–68 (2008). https://doi.org/10.1038/nm1666
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm1666
This article is cited by
-
Molecular mechanisms of coronary artery disease risk at the PDGFD locus
Nature Communications (2023)
-
The age of bone marrow dictates the clonality of smooth muscle-derived cells in atherosclerotic plaques
Nature Aging (2023)
-
Pericyte, but not astrocyte, hypoxia inducible factor-1 (HIF-1) drives hypoxia-induced vascular permeability in vivo
Fluids and Barriers of the CNS (2022)
-
Extracellular traps from activated vascular smooth muscle cells drive the progression of atherosclerosis
Nature Communications (2022)
-
Vascular derived endothelin receptor A controls endothelin-induced retinal ganglion cell death
Cell Death Discovery (2022)