Abstract
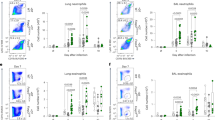
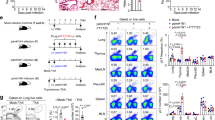
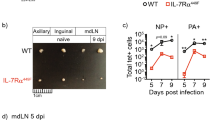
The complement cascade defines an important link between the innate and the specific immune system. Here we show that mice deficient for the third component of complement (C3−/− mice) are highly susceptible to primary infection with influenza virus. C3−/− mice showed delayed viral clearance and increased viral titers in lung, whereas mice deficient for complement receptors CR1 and CR2 (Cr2−/− mice) cleared the infection normally. Priming of T-helper cells and cytotoxic T cells (CTLs) in lung-draining lymph nodes was reduced, and the recruitment into the lung of virus-specific CD4+ and CD8+ effector T cells producing interferon-γ was severely impaired in C3−/− but not in Cr2−/− mice. Consequently, T-helper cell–dependent IgG responses were reduced in C3−/− mice but remained intact in Cr2−/− mice. These results demonstrate that complement induces specific immunity by promoting T-cell responses.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Carroll, M.C. & Prodeus, A.P. Linkages of innate and adaptive immunity. Curr. Opin. Immunol. 10, 36–40 (1998).
Brown, E.J. Complement receptors, adhesion, and phagocytosis. Infect. Agents Dis. 1, 63–70 (1992).
Muller-Eberhard, H.J. The membrane attack complex of complement. Annu. Rev. Immunol. 4, 503–528 (1986).
Thieblemont, N. et al. Triggering of complement receptors CR1 (CD35) and CR3 (CD11b/CD18) induces nuclear translocation of NF-kappa B (p50/p65) in human monocytes and enhances viral replication in HIV-infected monocytic cells. J. Immunol. 155, 4861–4867 (1995).
Carter, R.H., Spycher, M.O., Ng, Y.C., Hoffman, R. & Fearon, D.T. Synergistic interaction between complement receptor type 2 and membrane IgM on B lymphocytes. J. Immunol. 141, 457–463 (1988).
Carter, R.H. & Fearon, D.T. CD19: Lowering the threshold for antigen receptor stimulation of B lymphocytes. Science 256, 105–107 (1992).
van Noesel, C.J., Lankester, A.C. & van Lier, R.A. Dual antigen recognition by B cells. Immunol. Today 14, 8–11 (1993).
Fearon, D.T. & Carter, R.H. The CD19/CR2/TAPA-1 complex of B lymphocytes: linking natural to acquired immunity. Annu. Rev. Immunol. 13, 127–149 (1995).
Pepys, M.B. Role of complement in induction of antibody production In vivo. Effect of cobra factor and other C3-reactive agents on thymus-dependent and thymus-independent antibody responses. J. Exp. Med. 140, 126–145 (1974).
Engel, P. et al. Abnormal B lymphocyte development, activation and differentiation in mice that lack or overexpress the CD19 signal transduction molecule. Immunity 3, 39–50 (1995).
Ahearn, J.M. et al. Disruption of the Cr2 locus results in a reduction in B-1a cells and in an impaired B cell response to T-dependent antigen. Immunity 4, 251–262 (1996).
Croix, D.A. et al. Antibody response to a T-dependent antigen requires B cell expression of complement receptors. J. Exp. Med. 183, 1857–1864 (1996).
Dempsey, P.W., Allison, M.E., Akkaraju, S., Goodnow, C.C. & Fearon, D.T. C3d of complement as a molecular adjuvant: bridging innate and acquired immunity. Science 271, 348–350 (1996).
Papamichail, M. et al. Complement dependence of localisation of aggregated IgG in germinal centres. Scand J. Immunol. 4, 343–347 (1975).
Tew, J.G., Kosco, M.H., Burton, G.F. & Szakal, A.K. Follicular dendritic cells as accessory cells. Immunol. Rev. 117, 185–211 (1990).
Wessels, M.R. et al. Studies of group B streptococcal infection in mice deficient in complement component C3 or C4 demonstrate an essential role for complement in both innate and acquired immunity. Proc. Natl. Acad. Sci. USA 92, 11490–11494 (1995).
Reid, R.R. et al. Endotoxin shock in antibody-deficient mice: unraveling the role of natural antibody and complement in the clearance of lipopolysaccharide. J. Immunol. 159, 970–975 (1997).
Morgan, B. & Walport, M. Complement deficiency and disease. Immunol. Today 12, 301–306 (1991).
Daniels, C.A., Borsos, T., Rapp, H.J., Snyderman, R. & Notkins, A.L. Neutralization of sensitized virus by the fourth component of complement. Science 165, 508–509 (1969).
Linscott, W.D. & Levinson, W.E. Complement components required for virus neutralization by early immunoglobulin antibody. Proc. Natl. Acad. Sci. USA 64, 520–527 (1969).
Welsh, R.M. Jr, Lampert, P.W., Burner, P.A. & Oldstone, M.B. Antibody-complement interactions with purified lymphocytic choriomeningitis virus. Virology 73, 59–71 (1976).
Da Costa, X.J. et al. Humoral response to herpes simplex virus is complement-dependent. Proc. Natl. Acad. Sci. USA 96, 12708–12712 (1999).
Ochsenbein, A.F. et al. Protective T cell–independent antiviral antibody responses are dependent on complement. J. Exp. Med. 190, 1165–1174 (1999).
Graham, M.B. & Braciale, T.J. Resistance to and recovery from lethal influenza virus infection in B lymphocyte-deficient mice. J. Exp. Med. 186, 2063–2068 (1997).
Gerhard, W., Mozdzanowska, K., Furchner, M., Washko, G. & Maiese, K. Role of the B-cell response in recovery of mice from primary influenza virus infection. Immunol. Rev. 159, 95–103 (1997).
Doherty, P.C. et al. Effector CD4+ and CD8+ T-cell mechanisms in the control of respiratory virus infections. Immunol. Rev. 159, 105–117 (1997).
Fischer, M.B. et al. Regulation of the B cell response to T-dependent antigens by classical pathway complement. J. Immunol. 157, 549–556 (1996).
Bachmann, M.F. & Kundig, T.M. In vivo versus In vitro assays for assessment of T- and B-cell function. Curr. Opin. Immunol. 6, 320–326 (1994).
Bachmann, M.F., Kundig, T.M., Hengartner, H. & Zinkernagel, R.M. Regulation of IgG antibody titers by the amount persisting of immune-complexed antigen. Eur J. Immunol. 24, 2567–2570 (1994).
Huber, V.C., Lynch, J.M., Bucher, D.J., Le, J. & Metzger, D.W. Fc receptor-mediated phagocytosis makes a significant contribution to clearance of influenza virus infections. J. Immunol. 166, 7381–7388 (2001).
Guinamard, R., Okigaki, M., Schlessinger, J. & Ravetch, J. Absence of marginal zone B cells in Pyk-2-deficient mice defines their role in the humoral response. Nature Immunol. 1, 31–36 (2000).
Klein, M.A. et al. Complement facilitates early prion pathogenesis. Nature Med. 7, 488–492 (2001).
Mabbott, N.A., Bruce, M.E., Botto, M., Walport, M.J. & Pepys, M.B. Temporary depletion of complement component C3 or genetic deficiency of C1q significantly delays onset of scrapie. Nature Med. 7, 485–487 (2001).
Nataf, S., Davoust, N., Ames, R.S. & Barnum, S.R. Human T cells express the C5a receptor and are chemoattracted to C5a. J. Immunol. 162, 4018–4023 (1999).
Nataf, S., Carroll, S.L., Wetsel, R.A., Szalai, A.J. & Barnum, S.R. Attenuation of experimental autoimmune demyelination in complement-deficient mice. J. Immunol. 165, 5867–5873 (2000).
Wang, Y., Rollins, S.A., Madri, J.A. & Matis, L.A. Anti-C5 monoclonal antibody therapy prevents collagen-induced arthritis and ameliorates established disease. Proc. Natl. Acad. Sci. USA 92, 8955–8959 (1995).
Hopken, U.E., Lu, B., Gerard, N.P. & Gerard, C. The C5a chemoattractant receptor mediates mucosal defence to infection. Nature 383, 86–89 (1996).
Karp, C.L. et al. Identification of complement factor 5 as a susceptibility locus for experimental allergic asthma. Nature Immunol. 1, 221–226 (2000).
Czermak, B.J. et al. Protective effects of C5a blockade in sepsis. Nature Med. 5, 788–792 (1999).
Humbles, A.A. et al. A role for the C3a anaphylatoxin receptor in the effector phase of asthma. Nature 406, 998–1001 (2000).
Bachmann, M.F., Ecabert, B. & Kopf, M. Influenza virus: a novel method to assess viral and neutralizing antibody titers in vitro. J. Immunol. Methods 225, 105–111 (1999).
Ruedl, C., Bachmann, M.F. & Kopf, M. The antigen dose determines T helper subset development by regulation of CD40 ligand. Eur J. Immunol. 30, 2056–2064 (2000).
Kopf, M. et al. OX40-deficient mice are defective in Th cell proliferation but are competent in generating B cell and CTL Responses after virus infection. Immunity 11, 699–708 (1999).
Acknowledgements
We thank B. Ecabert and K. Lefrang for technical assistance, and G. Köhler for histology. A. Gallimore was supported by The Wellcome Trust (GR056527MA). The Basel Institute for Immunology was founded and supported by F. Hoffmann LaRoche.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Kopf, M., Abel, B., Gallimore, A. et al. Complement component C3 promotes T-cell priming and lung migration to control acute influenza virus infection. Nat Med 8, 373–378 (2002). https://doi.org/10.1038/nm0402-373
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nm0402-373
This article is cited by
-
Antibody-mediated NK cell activation as a correlate of immunity against influenza infection
Nature Communications (2023)
-
Influenza-associated thrombotic microangiopathies
Pediatric Nephrology (2018)
-
The complement system is also important in immunogenic cell death
Nature Reviews Immunology (2017)
-
iTRAQ-based quantitative proteomics reveals important host factors involved in the high pathogenicity of the H5N1 avian influenza virus in mice
Medical Microbiology and Immunology (2017)
-
Complement component 3 deficiency prolongs MHC-II disparate skin allograft survival by increasing the CD4+ CD25+ regulatory T cells population
Scientific Reports (2016)