Abstract
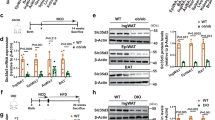
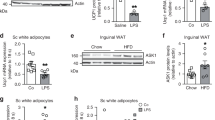
Beige adipocytes in white adipose tissue (WAT) are similar to classical brown adipocytes in that they can burn lipids to produce heat. Thus, an increase in beige adipocyte content in WAT browning would raise energy expenditure and reduce adiposity. Here we report that adipose-specific inactivation of Notch1 or its signaling mediator Rbpj in mice results in browning of WAT and elevated expression of uncoupling protein 1 (Ucp1), a key regulator of thermogenesis. Consequently, as compared to wild-type mice, Notch mutants exhibit elevated energy expenditure, better glucose tolerance and improved insulin sensitivity and are more resistant to high fat diet–induced obesity. By contrast, adipose-specific activation of Notch1 leads to the opposite phenotypes. At the molecular level, constitutive activation of Notch signaling inhibits, whereas Notch inhibition induces, Ppargc1a and Prdm16 transcription in white adipocytes. Notably, pharmacological inhibition of Notch signaling in obese mice ameliorates obesity, reduces blood glucose and increases Ucp1 expression in white fat. Therefore, Notch signaling may be therapeutically targeted to treat obesity and type 2 diabetes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Smith, R.E. Thermoregulatory and adaptive behavior of brown adipose tissue. Science 146, 1686–1689 (1964).
Nedergaard, J. & Cannon, B. The changed metabolic world with human brown adipose tissue: therapeutic visions. Cell Metab. 11, 268–272 (2010).
Rosen, E.D. & Spiegelman, B.M. Adipocytes as regulators of energy balance and glucose homeostasis. Nature 444, 847–853 (2006).
Tran, T.T. & Kahn, C.R. Transplantation of adipose tissue and stem cells: role in metabolism and disease. Nat. Rev. Endocrinol. 6, 195–213 (2010).
van Marken Lichtenbelt, W.D. et al. Cold-activated brown adipose tissue in healthy men. N. Engl. J. Med. 360, 1500–1508 (2009).
Saito, M. et al. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes 58, 1526–1531 (2009).
Virtanen, K.A. et al. Functional brown adipose tissue in healthy adults. N. Engl. J. Med. 360, 1518–1525 (2009).
Cypess, A.M. et al. Identification and importance of brown adipose tissue in adult humans. N. Engl. J. Med. 360, 1509–1517 (2009).
Zingaretti, M.C. et al. The presence of UCP1 demonstrates that metabolically active adipose tissue in the neck of adult humans truly represents brown adipose tissue. FASEB J. 23, 3113–3120 (2009).
Cypess, A.M. et al. Anatomical localization, gene expression profiling and functional characterization of adult human neck brown fat. Nat. Med. 19, 635–639 (2013).
Jespersen, N.Z. et al. A classical brown adipose tissue mRNA signature partly overlaps with brite in the supraclavicular region of adult humans. Cell Metab. 17, 798–805 (2013).
Lidell, M.E. et al. Evidence for two types of brown adipose tissue in humans. Nat. Med. 19, 631–634 (2013).
Schulz, T.J. et al. Brown-fat paucity due to impaired BMP signalling induces compensatory browning of white fat. Nature 495, 379–383 (2013).
Fisher, F.M. et al. FGF21 regulates PGC-1α and browning of white adipose tissues in adaptive thermogenesis. Genes Dev. 26, 271–281 (2012).
Ohno, H., Shinoda, K., Spiegelman, B.M. & Kajimura, S. PPARγ agonists induce a white-to-brown fat conversion through stabilization of PRDM16 protein. Cell Metab. 15, 395–404 (2012).
Seale, P. et al. Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. J. Clin. Invest. 121, 96–105 (2011).
Frontini, A. et al. White-to-brown transdifferentiation of omental adipocytes in patients affected by pheochromocytoma. Biochim. Biophys. Acta 1831, 950–959 (2013).
Wu, J. et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 150, 366–376 (2012).
Wang, Q.A., Tao, C., Gupta, R.K. & Scherer, P.E. Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat. Med. 19, 1338–1344 (2013).
Rosenwald, M., Perdikari, A., Rulicke, T. & Wolfrum, C. Bi-directional interconversion of brite and white adipocytes. Nat. Cell Biol. 15, 659–667 (2013).
Ye, L. et al. Fat cells directly sense temperature to activate thermogenesis. Proc. Natl. Acad. Sci. USA 110, 12480–12485 (2013).
Cao, L. et al. White to brown fat phenotypic switch induced by genetic and environmental activation of a hypothalamic-adipocyte axis. Cell Metab. 14, 324–338 (2011).
Shabalina, I.G. et al. UCP1 in brite/beige adipose tissue mitochondria is functionally thermogenic. Cell Reports 5, 1196–1203 (2013).
Pfannenberg, C. et al. Impact of age on the relationships of brown adipose tissue with sex and adiposity in humans. Diabetes 59, 1789–1793 (2010).
Ouellet, V. et al. Outdoor temperature, age, sex, body mass index, and diabetic status determine the prevalence, mass, and glucose-uptake activity of 18F-FDG–detected BAT in humans. J. Clin. Endocrinol. Metab. 96, 192–199 (2011).
Schroeter, E.H., Kisslinger, J.A. & Kopan, R. Notch-1 signalling requires ligand-induced proteolytic release of intracellular domain. Nature 393, 382–386 (1998).
Ross, D.A., Rao, P.K. & Kadesch, T. Dual roles for the Notch target gene Hes-1 in the differentiation of 3T3–L1 preadipocytes. Mol. Cell. Biol. 24, 3505–3513 (2004).
Garcés, C. et al. Notch-1 controls the expression of fatty acid–activated transcription factors and is required for adipogenesis. J. Biol. Chem. 272, 29729–29734 (1997).
Lai, P.Y., Tsai, C.B. & Tseng, M.J. Active form Notch4 promotes the proliferation and differentiation of 3T3–L1 preadipocytes. Biochem. Biophys. Res. Commun. 430, 1132–1139 (2013).
Urs, S. et al. Effect of soluble Jagged1-mediated inhibition of Notch signaling on proliferation and differentiation of an adipocyte progenitor cell model. Adipocyte 1, 46–57 (2012).
Vujovic, S., Henderson, S.R., Flanagan, A.M. & Clements, M.O. Inhibition of γ-secretases alters both proliferation and differentiation of mesenchymal stem cells. Cell Prolif. 40, 185–195 (2007).
Osathanon, T., Subbalekha, K., Sastravaha, P. & Pavasant, P. Notch signalling inhibits the adipogenic differentiation of single-cell–derived mesenchymal stem cell clones isolated from human adipose tissue. Cell Biol. Int. 36, 1161–1170 (2012).
Huang, Y. et al. γ-secretase inhibitor induces adipogenesis of adipose-derived stem cells by regulation of Notch and PPAR-γ. Cell Prolif. 43, 147–156 (2010).
Nichols, A.M. et al. Notch pathway is dispensable for adipocyte specification. Genesis 40, 40–44 (2004).
Stanford, K.I. et al. Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J. Clin. Invest. 123, 215–223 (2013).
Orava, J. et al. Different metabolic responses of human brown adipose tissue to activation by cold and insulin. Cell Metab. 14, 272–279 (2011).
Cannon, B. & Nedergaard, J. Nonshivering thermogenesis and its adequate measurement in metabolic studies. J. Exp. Biol. 214, 242–253 (2011).
Rosenbaum, M. & Leibel, R.L. The role of leptin in human physiology. N. Engl. J. Med. 341, 913–915 (1999).
Pajvani, U.B. et al. Inhibition of Notch uncouples Akt activation from hepatic lipid accumulation by decreasing mTorc1 stability. Nat. Med. 19, 1054–1060 (2013).
Weisberg, S.P. et al. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Invest. 112, 1796–1808 (2003).
Martens, K., Bottelbergs, A. & Baes, M. Ectopic recombination in the central and peripheral nervous system by aP2/FABP4-Cre mice: implications for metabolism research. FEBS Lett. 584, 1054–1058 (2010).
Urs, S., Harrington, A., Liaw, L. & Small, D. Selective expression of an aP2/fatty acid binding protein 4–Cre transgene in non-adipogenic tissues during embryonic development. Transgenic Res. 15, 647–653 (2006).
Mullican, S.E. et al. A novel adipose-specific gene deletion model demonstrates potential pitfalls of existing methods. Mol. Endocrinol. 27, 127–134 (2013).
Lee, K.Y. et al. Lessons on conditional gene targeting in mouse adipose tissue. Diabetes 62, 864–874 (2013).
Pajvani, U.B. et al. Inhibition of Notch signaling ameliorates insulin resistance in a FoxO1-dependent manner. Nat. Med. 17, 961–967 (2011).
Fukuda, D. et al. Notch ligand δ-like 4 blockade attenuates atherosclerosis and metabolic disorders. Proc. Natl. Acad. Sci. USA 109, E1868–E1877 (2012).
Eguchi, J. et al. Transcriptional control of adipose lipid handling by IRF4. Cell Metab. 13, 249–259 (2011).
Gerhart-Hines, Z. et al. The nuclear receptor Rev-erbα controls circadian thermogenic plasticity. Nature 503, 410–413 (2013).
Bray, S.J. Notch signalling: a simple pathway becomes complex. Nat. Rev. Mol. Cell Biol. 7, 678–689 (2006).
Iso, T. et al. HERP, a novel heterodimer partner of HES/Espl in Notch signaling. Mol. Cell. Biol. 21, 6080–6089 (2001).
Kurooka, H. & Honjo, T. Functional interaction between the mouse Notch1 intracellular region and histone acetyltransferases PCAF and GCN5. J. Biol. Chem. 275, 17211–17220 (2000).
Lerin, C. et al. GCN5 acetyltransferase complex controls glucose metabolism through transcriptional repression of PGC-1α. Cell Metab. 3, 429–438 (2006).
Kinameri, E. et al. Prdm proto-oncogene transcription factor family expression and interaction with the Notch-Hes pathway in mouse neurogenesis. PLoS ONE 3, e3859 (2008).
Schwanbeck, R., Martini, S., Bernoth, K. & Just, U. The Notch signaling pathway: molecular basis of cell context dependency. Eur. J. Cell Biol. 90, 572–581 (2011).
Battle, M. et al. Obesity induced a leptin-Notch signaling axis in breast cancer. Int. J. Cancer 134, 1605–1616 (2014).
Zecchini, V., Domaschenz, R., Winton, D. & Jones, P. Notch signaling regulates the differentiation of post-mitotic intestinal epithelial cells. Genes Dev. 19, 1686–1691 (2005).
Liu, W. et al. A heterogeneous lineage origin underlies the phenotypic and molecular differences of white and beige adipocytes. J. Cell Sci. 126, 3527–3532 (2013).
Han, H. et al. Inducible gene knockout of transcription factor recombination signal binding protein-J reveals its essential role in T versus B lineage decision. Int. Immunol. 14, 637–645 (2002).
Yang, X. et al. Notch activation induces apoptosis in neural progenitor cells through a p53-dependent pathway. Dev. Biol. 269, 81–94 (2004).
He, W. et al. Adipose-specific peroxisome proliferator–activated receptor γ knockout causes insulin resistance in fat and liver but not in muscle. Proc. Natl. Acad. Sci. USA 100, 15712–15717 (2003).
Coleman, D.L. & Hummel, K.P. The influence of genetic background on the expression of the obese (Ob) gene in the mouse. Diabetologia 9, 287–293 (1973).
Murtaugh, L.C., Stanger, B.Z., Kwan, K.M. & Melton, D.A. Notch signaling controls multiple steps of pancreatic differentiation. Proc. Natl. Acad. Sci. USA 100, 14920–14925 (2003).
Milano, J. et al. Modulation of notch processing by γ-secretase inhibitors causes intestinal goblet cell metaplasia and induction of genes known to specify gut secretory lineage differentiation. Toxicol. Sci. 82, 341–358 (2004).
Wen, Y. et al. Constitutive Notch activation upregulates Pax7 and promotes the self-renewal of skeletal muscle satellite cells. Mol. Cell. Biol. 32, 2300–2311 (2012).
Shan, T., Liang, X., Bi, P. & Kuang, S. Myostatin knockout drives browning of white adipose tissue through activating the AMPK-PGC1α-Fndc5 pathway in muscle. FASEB J. 27, 1981–1989 (2013).
Demehri, S., Turkoz, A. & Kopan, R. Epidermal Notch1 loss promotes skin tumorigenesis by impacting the stromal microenvironment. Cancer Cell 16, 55–66 (2009).
Gao, J. et al. Hedgehog signaling is dispensable for adult hematopoietic stem cell function. Cell Stem Cell 4, 548–558 (2009).
Handschin, C., Rhee, J., Lin, J., Tarr, P.T. & Spiegelman, B.M. An autoregulatory loop controls peroxisome proliferator–activated receptor γ coactivator 1α expression in muscle. Proc. Natl. Acad. Sci. USA 100, 7111–7116 (2003).
Acknowledgements
We thank T. Honjo (Kyoto University, Japan) for providing the Rbpjflox/flox mice; Dow AgroScience for providing Methocel E4M reagent; K. Ajuwon for providing access to the calorimetry facility; S. Koser and S. Donkin for assistance with luciferase assays; D. Zhou for Odyssey imaging facility support; T. Wiegand and C. Bain for assistance with histology; J. Wu and S. Hobaugh for maintaining mouse colonies; and members of the Kuang laboratory for comments. This work was partially supported by a grant from the US National Institutes of Health (R01AR060652 to S.K.).
Author information
Authors and Affiliations
Contributions
P.B. and S.K. conceived the project, designed the experiments and prepared the manuscript. P.B., T.S., W.L., F.Y., X.Y., X.-R.L., J.W. and J.L. performed the experiments. N.C. provided reagents. P.B., T.S., W.L., X.L. and S.K. analyzed the data.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–7 and Supplementary Table 1 (PDF 823 kb)
Supplementary Video 1
Movement of WT (left) and aNotch1 (right) mice in the new cages. (MOV 3831 kb)
Rights and permissions
About this article
Cite this article
Bi, P., Shan, T., Liu, W. et al. Inhibition of Notch signaling promotes browning of white adipose tissue and ameliorates obesity. Nat Med 20, 911–918 (2014). https://doi.org/10.1038/nm.3615
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3615
This article is cited by
-
Modulation of apoptosis and Inflammasome activation in chondrocytes: co-regulatory role of Chlorogenic acid
Cell Communication and Signaling (2024)
-
SLC35D3 promotes white adipose tissue browning to ameliorate obesity by NOTCH signaling
Nature Communications (2023)
-
TGFBI remodels adipose metabolism by regulating the Notch-1 signaling pathway
Experimental & Molecular Medicine (2023)
-
Notch signaling regulates a metabolic switch through inhibiting PGC-1α and mitochondrial biogenesis in dedifferentiated liposarcoma
Oncogene (2023)
-
Endothelial Notch1 signaling in white adipose tissue promotes cancer cachexia
Nature Cancer (2023)