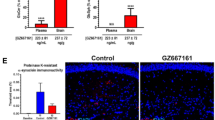
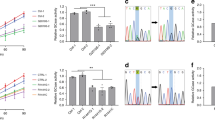
Abstract
Gaucher's disease (GD), an inherited metabolic disorder caused by mutations in the glucocerebrosidase gene (GBA), is the most common lysosomal storage disease1. Heterozygous mutations in GBA are a major risk factor for Parkinson's disease2. GD is divided into three clinical subtypes based on the absence (type 1) or presence (types 2 and 3) of neurological signs. Type 1 GD was the first lysosomal storage disease (LSD) for which enzyme therapy became available, and although infusions of recombinant glucocerebrosidase (GCase) ameliorate the systemic effects of GD, the lack of efficacy for the neurological manifestations, along with the considerable expense3 and inconvenience of enzyme therapy for patients, renders the search for alternative or complementary therapies paramount. Glucosylceramide and glucosylsphingosine accumulation in the brain leads to massive neuronal loss in patients with neuronopathic GD (nGD)4 and in nGD mouse models5,6,7. However, the mode of neuronal death is not known. Here, we show that modulating the receptor-interacting protein kinase-3 (Ripk3) pathway markedly improves neurological and systemic disease in a mouse model of GD. Notably, Ripk3 deficiency substantially improved the clinical course of GD mice, with increased survival and motor coordination and salutary effects on cerebral as well as hepatic injury.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Futerman, A.H. & van Meer, G. The cell biology of lysosomal storage disorders. Nat. Rev. Mol. Cell Biol. 5, 554–565 (2004).
Eblan, M.J., Walker, J.M. & Sidransky, E. The glucocerebrosidase gene and Parkinson′s disease in Ashkenazi Jews. N. Engl. J. Med. 352, 728–731 (2005).
Cox, T.M. Competing for the treasure in exceptions. Am. J. Hematol. 88, 163–165 (2013).
Wong, K. et al. Neuropathology provides clues to the pathophysiology of Gaucher disease. Mol. Genet. Metab. 82, 192–207 (2004).
Farfel-Becker, T. et al. Spatial and temporal correlation between neuron loss and neuroinflammation in a mouse model of neuronopathic Gaucher disease. Hum. Mol. Genet. 20, 1375–1386 (2011).
Vitner, E.B., Farfel-Becker, T., Eilam, R., Biton, I. & Futerman, A.H. Contribution of brain inflammation to neuronal cell death in neuronopathic forms of Gaucher′s disease. Brain 135, 1724–1735 (2012).
Enquist, I.B. et al. Murine models of acute neuronopathic Gaucher disease. Proc. Natl. Acad. Sci. USA 104, 17483–17488 (2007).
Kanfer, J.N., Legler, G., Sullivan, J., Raghavan, S.S. & Mumford, R.A. The Gaucher mouse. Biochem. Biophys. Res. Commun. 67, 85–90 (1975).
Vandenabeele, P., Galluzzi, L., Vanden Berghe, T. & Kroemer, G. Molecular mechanisms of necroptosis: an ordered cellular explosion. Nat. Rev. Mol. Cell Biol. 11, 700–714 (2010).
Feoktistova, M. et al. cIAPs block Ripoptosome formation, a RIP1/caspase-8 containing intracellular cell death complex differentially regulated by cFLIP isoforms. Mol. Cell 43, 449–463 (2011).
Tenev, T. et al. The Ripoptosome, a signaling platform that assembles in response to genotoxic stress and loss of IAPs. Mol. Cell 43, 432–448 (2011).
Upton, J.W., Kaiser, W.J. & Mocarski, E.S. Virus inhibition of RIP3-dependent necrosis. Cell Host Microbe 7, 302–313 (2010).
Lin, Y., Devin, A., Rodriguez, Y. & Liu, Z.G. Cleavage of the death domain kinase RIP by caspase-8 prompts TNF-induced apoptosis. Genes Dev. 13, 2514–2526 (1999).
Zhang, D.-W. et al. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science 325, 332–336 (2009).
Silke, J. & Strasser, A. The FLIP side of life. Sci. Signal. 6, pe2 (2013).
Oberst, A. et al. Catalytic activity of the caspase-8-FLIP(L) complex inhibits RIPK3-dependent necrosis. Nature 471, 363–367 (2011).
Trichonas, G. et al. Receptor interacting protein kinases mediate retinal detachment-induced photoreceptor necrosis and compensate for inhibition of apoptosis. Proc. Natl. Acad. Sci. USA 107, 21695–21700 (2010).
Lin, J. et al. A role of RIP3-mediated macrophage necrosis in atherosclerosis development. Cell Rep. 3, 200–210 (2013).
Cho, Y.S. et al. Phosphorylation-driven assembly of the RIP1–RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell 137, 1112–1123 (2009).
Duprez, L. et al. RIP kinase–dependent necrosis drives lethal systemic inflammatory response syndrome. Immunity 35, 908–918 (2011).
Roychowdhury, S., McMullen, M.R., Pisano, S.G., Liu, X. & Nagy, L.E. Absence of receptor interacting protein kinase 3 prevents ethanol-induced liver injury. Hepatology 57, 1773–1783 (2013).
Kang, T.-B., Yang, S.-H., Toth, B., Kovalenko, A. & Wallach, D. Caspase-8 blocks kinase RIPK3-mediated activation of the NLRP3 inflammasome. Immunity 38, 27–40 (2013).
Kovalenko, A. et al. Caspase-8 deficiency in epidermal keratinocytes triggers an inflammatory skin disease. J. Exp. Med. 206, 2161–2177 (2009).
Lee, P. et al. Dynamic expression of epidermal caspase 8 simulates a wound healing response. Nature 458, 519–523 (2009).
Kaiser, W.J. et al. RIP3 mediates the embryonic lethality of caspase-8–deficient mice. Nature 471, 368–372 (2011).
Yang, Y., Ma, J., Chen, Y. & Wu, M. Nucleocytoplasmic shuttling of receptor-interacting protein 3 (RIP3): identification of novel nuclear export and import signals in RIP3. J. Biol. Chem. 279, 38820–38829 (2004).
Suzuki, K. & Taniike, M. Murine model of genetic demyelinating disease: the twitcher mouse. Microsc. Res. Tech. 32, 204–214 (1995).
Vitner, E.B., Platt, F.M. & Futerman, A.H. Common and uncommon pathogenic cascades in lysosomal storage diseases. J. Biol. Chem. 285, 20423–20427 (2010).
Vitner, E.B. et al. Altered expression and distribution of cathepsins in neuronopathic forms of Gaucher disease and in other sphingolipidoses. Hum. Mol. Genet. 19, 3583–3590 (2010).
Kelliher, M.A. et al. The death domain kinase RIP mediates the TNF-induced NF-κB signal. Immunity 8, 297–303 (1998).
Newton, K., Sun, X. & Dixit, V.M. Kinase RIP3 is dispensable for normal NF-κ Bs, signaling by the B-cell and T-cell receptors, tumor necrosis factor receptor 1, and Toll-like receptors 2 and 4. Mol. Cell. Biol. 24, 1464–1469 (2004).
Farfel-Becker, T., Vitner, E.B. & Futerman, A.H. Animal models for Gaucher disease research. Dis. Model. Mech. 4, 746–752 (2011).
Wallach, D., Kovalenko, A. & Kang, T.-B. 'Necrosome'-induced inflammation: must cells die for it? Trends Immunol. 32, 505–509 (2011).
Chavez-Valdez, R., Martin, L.J., Flock, D.L. & Northington, F.J. Necrostatin-1 attenuates mitochondrial dysfunction in neurons and astrocytes following neonatal hypoxia-ischemia. Neuroscience 219, 192–203 (2012).
Rosenbaum, D.M. et al. Necroptosis, a novel form of caspase-independent cell death, contributes to neuronal damage in a retinal ischemia-reperfusion injury model. J. Neurosci. Res. 88, 1569–1576 (2010).
You, Z. et al. Necrostatin-1 reduces histopathology and improves functional outcome after controlled cortical impact in mice. J. Cereb. Blood Flow Metab. 28, 1564–1573 (2008).
Jagtap, P.G. et al. Structure-activity relationship study of tricyclic necroptosis inhibitors. J. Med. Chem. 50, 1886–1895 (2007).
Zhu, S., Zhang, Y., Bai, G. & Li, H. Necrostatin-1 ameliorates symptoms in R6/2 transgenic mouse model of Huntington′s disease. Cell Death Dis. 2, e115 (2011).
Kaiser, W.J. et al. Toll-like receptor 3–mediated necrosis via TRIF, RIP3 and MLKL. J. Biol. Chem. 288, 31268–31279 (2013).
Sun, L. et al. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell 148, 213–227 (2012).
Farfel-Becker, T. et al. No evidence for activation of the unfolded protein response in neuronopathic models of Gaucher disease. Hum. Mol. Genet. 18, 1482–1488 (2009).
Sango, K. et al. Mouse models of Tay-Sachs and Sandhoff diseases differ in neurologic phenotype and ganglioside metabolism. Nat. Genet. 11, 170–176 (1995).
Hahn, C.N. et al. Generalized CNS disease and massive GM1-ganglioside accumulation in mice defective in lysosomal acid β-galactosidase. Hum. Mol. Genet. 6, 205–211 (1997).
Pentchev, P.G. et al. A genetic storage disorder in BALB/C mice with a metabolic block in esterification of exogenous cholesterol. J. Biol. Chem. 259, 5784–5791 (1984).
Narayan, N. et al. The NAD-dependent deacetylase SIRT2 is required for programmed necrosis. Nature 492, 199–204 (2012).
Lopez, M.E., Klein, A.D., Dimbil, U.J. & Scott, M.P. Anatomically defined neuron-based rescue of neurodegenerative Niemann-Pick type C disorder. J. Neurosci. 31, 4367–4378 (2011).
van Raam, B.J., Ehrnhoefer, D.E., Hayden, M.R. & Salvesen, G.S. Intrinsic cleavage of receptor-interacting protein kinase-1 by caspase-6. Cell Death Differ. 20, 86–96 (2013).
Hung, T. et al. Extensive and coordinated transcription of noncoding RNAs within cell-cycle promoters. Nat. Genet. 43, 621–629 (2011).
Han, J., Sridevi, P., Ramirez, M., Ludwig, K.J. & Wang, J.Y.J. β-Catenin-dependent lysosomal targeting of internalized tumor necrosis factor-α suppresses caspase-8 activation in apoptosis-resistant colon cancer cells. Mol. Biol. Cell 24, 465–473 (2013).
Acknowledgements
We thank B. Cachón-González (University of Cambridge) for providing twitcher mouse tissue, D. Wallach (Weizmann Institute of Science, Israel) for providing Tnf and Ripk3 knockout mice, R. Schiffmann (Baylor Research Institute) for postmortem human brain tissue, V. Kiss (Weizmann Institute of Science) for help with fluorescence microscopy and N. Platt (University of Oxford) for helpful comments. This work was supported by the Children′s Gaucher Research Fund. A.H.F. is the incumbent of an endowed professorial chair supported by the Joseph Meyerhoff family. F.M.P. is a Royal Society Wolfson Research Merit Award holder.
Author information
Authors and Affiliations
Contributions
E.B.V. and R.S. planned and performed most of the experiments and wrote the manuscript. T.F.-B., A.M., M.A. and A.D.K. performed specific experiments, and F.M.P. and T.M.C. participated in experimental design and provided tissues. A.H.F. participated in experimental design, supervised and funded the project and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figure 1 (PDF 107 kb)
Rights and permissions
About this article
Cite this article
Vitner, E., Salomon, R., Farfel-Becker, T. et al. RIPK3 as a potential therapeutic target for Gaucher's disease. Nat Med 20, 204–208 (2014). https://doi.org/10.1038/nm.3449
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3449
This article is cited by
-
Innate immune sensing of lysosomal dysfunction drives multiple lysosomal storage disorders
Nature Cell Biology (2024)
-
Mediators of necroptosis: from cell death to metabolic regulation
EMBO Molecular Medicine (2024)
-
A guide to cell death pathways
Nature Reviews Molecular Cell Biology (2023)
-
Inflammation and immune dysfunction in Parkinson disease
Nature Reviews Immunology (2022)
-
Glycosphingolipid metabolism and its role in ageing and Parkinson’s disease
Glycoconjugate Journal (2022)