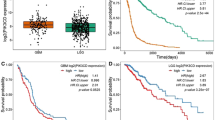
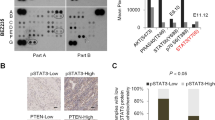
Abstract
In glioblastoma, phosphatidylinositol 3-kinase (PI3K) signaling is frequently activated by loss of the tumor suppressor phosphatase and tensin homolog (PTEN)1. However, it is not known whether inhibiting PI3K represents a selective and effective approach for treatment. We interrogated large databases and found that sonic hedgehog (SHH) signaling is activated in PTEN-deficient glioblastoma. We demonstrate that the SHH and PI3K pathways synergize to promote tumor growth and viability in human PTEN-deficient glioblastomas. A combination of PI3K and SHH signaling inhibitors not only suppressed the activation of both pathways but also abrogated S6 kinase (S6K) signaling. Accordingly, targeting both pathways simultaneously resulted in mitotic catastrophe and tumor apoptosis and markedly reduced the growth of PTEN-deficient glioblastomas in vitro and in vivo. The drugs tested here appear to be safe in humans; therefore, this combination may provide a new targeted treatment for glioblastoma.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Cerami, E., Demir, E., Schultz, N., Taylor, B.S. & Sander, C. Automated network analysis identifies core pathways in glioblastoma. PLoS ONE 5, e8918 (2010).
Stupp, R. et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 352, 987–996 (2005).
Quant, E.C. & Wen, P.Y. Novel medical therapeutics in glioblastomas, including targeted molecular therapies, current and future clinical trials. Neuroimaging Clin. N. Am. 20, 425–448 (2010).
Koul, D. et al. Antitumor activity of NVP-BKM120—a selective pan class I PI3 kinase inhibitor showed differential forms of cell death based on p53 status of glioma cells. Clin. Cancer Res. 18, 184–195 (2012).
Maira, S.M. et al. Identification and characterization of NVP-BKM120, an orally available pan-class I PI3-kinase inhibitor. Mol. Cancer Ther. 11, 317–328 (2012).
Phillips, H.S. et al. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell 9, 157–173 (2006).
Cancer Genome Atlas Research Network. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature 455, 1061–1068 (2008).
Pandita, A., Aldape, K.D., Zadeh, G., Guha, A. & James, C.D. Contrasting in vivo and in vitro fates of glioblastoma cell subpopulations with amplified EGFR. Genes Chromosom. Cancer 39, 29–36 (2004).
Venere, M., Fine, H.A., Dirks, P.B. & Rich, J.N. Cancer stem cells in gliomas: identifying and understanding the apex cell in cancer's hierarchy. Glia 59, 1148–1154 (2011).
Pan, S. et al. Discovery of NVP-LDE225, a potent and selective Smoothened antagonist. ACS Med. Chem. Lett. 1, 130–134 (2010).
Folkes, A.J. et al. The identification of 2-(1H-indazol-4-yl)-6-(4-methanesulfonyl-piperazin-1-ylmethyl)-4-morpholin-4-yl-t hieno[3,2-d]pyrimidine (GDC-0941) as a potent, selective, orally bioavailable inhibitor of class I PI3 kinase for the treatment of cancer. J. Med. Chem. 51, 5522–5532 (2008).
Pollard, S.M. et al. Glioma stem cell lines expanded in adherent culture have tumor-specific phenotypes and are suitable for chemical and genetic screens. Cell Stem Cell 4, 568–580 (2009).
Rubin, J.B. et al. A small-molecule antagonist of CXCR4 inhibits intracranial growth of primary brain tumors. Proc. Natl. Acad. Sci. USA 100, 13513–13518 (2003).
Vakifahmetoglu, H., Olsson, M. & Zhivotovsky, B. Death through a tragedy: mitotic catastrophe. Cell Death Differ. 15, 1153–1162 (2008).
Manning, B.D. Balancing Akt with S6K: implications for both metabolic diseases and tumorigenesis. J. Cell Biol. 167, 399–403 (2004).
Dancey, J.E. Inhibitors of the mammalian target of rapamycin. Expert Opin. Investig. Drugs 14, 313–328 (2005).
Tandon, P. et al. Requirement for ribosomal protein S6 kinase 1 to mediate glycolysis and apoptosis resistance induced by Pten deficiency. Proc. Natl. Acad. Sci. USA 108, 2361–2365 (2011).
Chen, J.K., Taipale, J., Young, K.E., Maiti, T. & Beachy, P.A. Small molecule modulation of Smoothened activity. Proc. Natl. Acad. Sci. USA 99, 14071–14076 (2002).
Choi, H.N. et al. Inhibition of S6K1 enhances glucose deprivation-induced cell death via downregulation of anti-apoptotic proteins in MCF-7 breast cancer cells. Biochem. Biophys. Res. Commun. 432, 123–128 (2013).
Kenney, A.M. & Rowitch, D.H. Sonic hedgehog promotes G(1) cyclin expression and sustained cell cycle progression in mammalian neuronal precursors. Mol. Cell. Biol. 20, 9055–9067 (2000).
Liang, J. & Slingerland, J.M. Multiple roles of the PI3K/PKB (Akt) pathway in cell cycle progression. Cell Cycle 2, 339–345 (2003).
Barbus, S. et al. Differential retinoic acid signaling in tumors of long- and short-term glioblastoma survivors. J. Natl. Cancer Inst. 103, 598–606 (2011).
Lee, E.Y. et al. Hedgehog pathway–regulated gene networks in cerebellum development and tumorigenesis. Proc. Natl. Acad. Sci. USA 107, 9736–9741 (2010).
Masi, A. et al. hERG1 channels are overexpressed in glioblastoma multiforme and modulate VEGF secretion in glioblastoma cell lines. Br. J. Cancer 93, 781–792 (2005).
Workman, P., Clarke, P.A., Raynaud, F.I. & van Montfort, R.L. Drugging the PI3 kinome: from chemical tools to drugs in the clinic. Cancer Res. 70, 2146–2157 (2010).
Xu, Q., Yuan, X., Liu, G., Black, K.L. & Yu, J.S. Hedgehog signaling regulates brain tumor-initiating cell proliferation and portends shorter survival for patients with PTEN-coexpressing glioblastomas. Stem Cells 26, 3018–3026 (2008).
Becher, O.J. et al. Gli activity correlates with tumor grade in platelet-derived growth factor–induced gliomas. Cancer Res. 68, 2241–2249 (2008).
Castellino, R.C. et al. Heterozygosity for Pten promotes tumorigenesis in a mouse model of medulloblastoma. PLoS ONE 5, e10849 (2010).
Buonamici, S. et al. Interfering with resistance to smoothened antagonists by inhibition of the PI3K pathway in medulloblastoma. Sci. Transl. Med. 2, 51ra70 (2010).
Riobó, N.A., Lu, K., Ai, X., Haines, G.M. & Emerson, C.P. Jr. Phosphoinositide 3-kinase and Akt are essential for Sonic Hedgehog signaling. Proc. Natl. Acad. Sci. USA 103, 4505–4510 (2006).
Kenney, A.M., Widlund, H.R. & Rowitch, D.H. Hedgehog and PI-3 kinase signaling converge on Nmyc1 to promote cell cycle progression in cerebellar neuronal precursors. Development 131, 217–228 (2004).
Mizuarai, S., Kawagishi, A. & Kotani, H. Inhibition of p70S6K2 down-regulates Hedgehog/GLI pathway in non-small cell lung cancer cell lines. Mol. Cancer 8, 44 (2009).
Evangelista, M. et al. Kinome siRNA screen identifies regulators of ciliogenesis and hedgehog signal transduction. Sci. Signal. 1, ra7 (2008).
Wang, Y. et al. The crosstalk of mTOR/S6K1 and Hedgehog pathways. Cancer Cell 21, 374–387 (2012).
Eimer, S. et al. Cyclopamine cooperates with EGFR inhibition to deplete stem-like cancer cells in glioblastoma-derived spheroid cultures. Neuro-oncol. 14, 1441–1451 (2012).
Ferruzzi, P. et al. In vitro and in vivo characterization of a novel Hedgehog signaling antagonist in human glioblastoma cell lines. Int. J. Cancer 131, E33–E44 (2012).
Hsieh, A., Ellsworth, R. & Hsieh, D. Hedgehog/GLI1 regulates IGF dependent malignant behaviors in glioma stem cells. J. Cell. Physiol. 226, 1118–1127 (2011).
Santoni, M. et al. Essential role of Gli proteins in glioblastoma multiforme. Curr. Protein Pept. Sci. 14, 133–140 (2013).
Tremblay, M.R., McGovern, K., Read, M.A. & Castro, A.C. New developments in the discovery of small molecule Hedgehog pathway antagonists. Curr. Opin. Chem. Biol. 14, 428–435 (2010).
Acknowledgements
We thank V. Monrose and K. Jones for technical assistance and C. Stiles for helpful discussions. We thank N. Gray (Dana-Farber Cancer Institute) for PF-4708671 and C. David James (University of California San Francisco) for the hBT70 and hBT75 lines. This work was supported by an Austrian Programme for Advanced Research and Technology (APART) fellowship of the Austrian Academy of Sciences (M.G.F.), grants from the US National Institutes of Health (R.A.S.) and Novartis Pharmaceuticals.
Author information
Authors and Affiliations
Contributions
M.G.F., S.K.D., E.P. and R.A.S. wrote the manuscript with input from all authors. M.G.F., S.K.D., E.P., J.C., M.F.P.-M. and Yu Sun executed the in vitro studies and analyzed the in vitro and in vivo studies. A.L.K., Yanping Sun, Y.F., J.F.K., S.B. and M.D. performed the in vivo studies. M.A.T., K.L.L. and S.R. generated GBM lines and analyzed tumor pathology. D.S.S. and N.R. initiated the studies. B.T. and R.B. analyzed databases. J.Z. and Q.W. provided shRNA specific to PTEN.
Corresponding author
Ethics declarations
Competing interests
This work was supported in part by grants from Novartis Institute of BioMedical research. J.F.K. is an employee of, and R.A.S. had a consulting agreement with, Novartis Institute of BioMedical Research.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–3 (PDF 6026 kb)
Supplementary Table 1
Supplementary Table 1 (XLS 514 kb)
Rights and permissions
About this article
Cite this article
Gruber Filbin, M., Dabral, S., Pazyra-Murphy, M. et al. Coordinate activation of Shh and PI3K signaling in PTEN-deficient glioblastoma: new therapeutic opportunities. Nat Med 19, 1518–1523 (2013). https://doi.org/10.1038/nm.3328
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3328
This article is cited by
-
A novel protein encoded by circular SMO RNA is essential for Hedgehog signaling activation and glioblastoma tumorigenicity
Genome Biology (2021)
-
Knockdown of DEPDC1B inhibits the development of glioblastoma
Cancer Cell International (2020)
-
Inhibition of sonic hedgehog and PI3K/Akt/mTOR pathways cooperate in suppressing survival, self-renewal and tumorigenic potential of glioblastoma-initiating cells
Molecular and Cellular Biochemistry (2019)
-
PTEN lipid phosphatase inactivation links the hippo and PI3K/Akt pathways to induce gastric tumorigenesis
Journal of Experimental & Clinical Cancer Research (2018)
-
A synthetic combinatorial approach to disabling deviant Hedgehog signaling
Scientific Reports (2018)