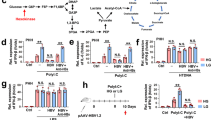
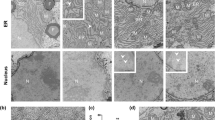
Abstract
In most adult humans, hepatitis B is a self-limiting disease leading to life-long protective immunity, which is the consequence of a robust adaptive immune response occurring weeks after hepatitis B virus (HBV) infection. Notably, HBV-specific T cells can be detected shortly after infection, but the mechanisms underlying this early immune priming and its consequences for subsequent control of viral replication are poorly understood. Using primary human and mouse hepatocytes and mouse models of transgenic and adenoviral HBV expression, we show that HBV-expressing hepatocytes produce endoplasmic reticulum (ER)-associated endogenous antigenic lipids including lysophospholipids that are generated by HBV-induced secretory phospholipases and that lead to activation of natural killer T (NKT) cells. The absence of NKT cells or CD1d or a defect in ER-associated transfer of lipids onto CD1d results in diminished HBV-specific T and B cell responses and delayed viral control in mice. NKT cells may therefore contribute to control of HBV infection through sensing of HBV-induced modified self-lipids.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Thimme, R. et al. CD8+ T cells mediate viral clearance and disease pathogenesis during acute hepatitis B virus infection. J. Virol. 77, 68–76 (2003).
Yeo, W. et al. Hepatitis B virus reactivation in lymphoma patients with prior resolved hepatitis B undergoing anticancer therapy with or without rituximab. J. Clin. Oncol. 27, 605–611 (2009).
Esteve, M. et al. Chronic hepatitis B reactivation following infliximab therapy in Crohn's disease patients: need for primary prophylaxis. Gut 53, 1363–1365 (2004).
Calabrese, L.H., Zein, N.N. & Vassilopoulos, D. Hepatitis B virus (HBV) reactivation with immunosuppressive therapy in rheumatic diseases: assessment and preventive strategies. Ann. Rheum. Dis. 65, 983–989 (2006).
Thio, C.L. et al. HIV-1, hepatitis B virus, and risk of liver-related mortality in the Multicenter Cohort Study (MACS). Lancet 360, 1921–1926 (2002).
Guidotti, L.G. & Chisari, F.V. Immunobiology and pathogenesis of viral hepatitis. Annu. Rev. Pathol. 1, 23–61 (2006).
Bendelac, A., Savage, P.B. & Teyton, L. The biology of NKT cells. Annu. Rev. Immunol. 25, 297–336 (2007).
Tupin, E., Kinjo, Y. & Kronenberg, M. The unique role of natural killer T cells in the response to microorganisms. Nat. Rev. Microbiol. 5, 405–417 (2007).
Wieland, S., Thimme, R., Purcell, R.H. & Chisari, F.V. Genomic analysis of the host response to hepatitis B virus infection. Proc. Natl. Acad. Sci. USA 101, 6669–6674 (2004).
Fisicaro, P. et al. Early kinetics of innate and adaptive immune responses during hepatitis B virus infection. Gut 58, 974–982 (2009).
Webster, G.J. et al. Incubation phase of acute hepatitis B in man: dynamic of cellular immune mechanisms. Hepatology 32, 1117–1124 (2000).
Guy, C.S., Mulrooney-Cousins, P.M., Churchill, N.D. & Michalak, T.I. Intrahepatic expression of genes affiliated with innate and adaptive immune responses immediately after invasion and during acute infection with woodchuck hepadnavirus. J. Virol. 82, 8579–8591 (2008).
Kakimi, K., Guidotti, L.G., Koezuka, Y. & Chisari, F.V. Natural killer T cell activation inhibits hepatitis B virus replication in vivo. J. Exp. Med. 192, 921–930 (2000).
Isogawa, M., Kakimi, K., Kamamoto, H., Protzer, U. & Chisari, F.V. Differential dynamics of the peripheral and intrahepatic cytotoxic T lymphocyte response to hepatitis B surface antigen. Virology 333, 293–300 (2005).
Sprinzl, M.F., Oberwinkler, H., Schaller, H. & Protzer, U. Transfer of hepatitis B virus genome by adenovirus vectors into cultured cells and mice: crossing the species barrier. J. Virol. 75, 5108–5118 (2001).
von Freyend, M.J. et al. Sequential control of hepatitis B virus in a mouse model of acute, self-resolving hepatitis B. J. Viral Hepat. 18, 216–226 (2011).
Guidotti, L.G. et al. Viral clearance without destruction of infected cells during acute HBV infection. Science 284, 825–829 (1999).
Publicover, J. et al. IL-21 is pivotal in determining age-dependent effectiveness of immune responses in a mouse model of human hepatitis B. J. Clin. Invest. 121, 1154–1162 (2011).
Baron, J.L. et al. Activation of a nonclassical NKT cell subset in a transgenic mouse model of hepatitis B virus infection. Immunity 16, 583–594 (2002).
Vilarinho, S., Ogasawara, K., Nishimura, S., Lanier, L.L. & Baron, J.L. Blockade of NKG2D on NKT cells prevents hepatitis and the acute immune response to hepatitis B virus. Proc. Natl. Acad. Sci. USA 104, 18187–18192 (2007).
Brozovic, S. et al. CD1d function is regulated by microsomal triglyceride transfer protein. Nat. Med. 10, 535–539 (2004).
Dougan, S.K., Rava, P., Hussain, M.M. & Blumberg, R.S. MTP regulated by an alternate promoter is essential for NKT cell development. J. Exp. Med. 204, 533–545 (2007).
Dougan, S.K. et al. Microsomal triglyceride transfer protein lipidation and control of CD1d on antigen-presenting cells. J. Exp. Med. 202, 529–539 (2005).
Kaser, A. et al. Microsomal triglyceride transfer protein regulates endogenous and exogenous antigen presentation by group 1 CD1 molecules. Eur. J. Immunol. 38, 2351–2359 (2008).
Zeissig, S. et al. Primary deficiency of microsomal triglyceride transfer protein in human abetalipoproteinemia is associated with loss of CD1 function. J. Clin. Invest. 120, 2889–2899 (2010).
Khatun, I. et al. Phospholipid transfer activity of microsomal triglyceride transfer protein produces apolipoprotein B and reduces hepatosteatosis while maintaining low plasma lipids in mice. Hepatology 55, 1356–1368 (2012).
Ganem, D. & Prince, A.M. Hepatitis B virus infection—natural history and clinical consequences. N. Engl. J. Med. 350, 1118–1129 (2004).
Patient, R., Hourioux, C. & Roingeard, P. Morphogenesis of hepatitis B virus and its subviral envelope particles. Cell. Microbiol. 11, 1561–1570 (2009).
Satoh, O., Umeda, M., Imai, H., Tunoo, H. & Inoue, K. Lipid composition of hepatitis B virus surface antigen particles and the particle-producing human hepatoma cell lines. J. Lipid Res. 31, 1293–1300 (1990).
Arrenberg, P., Halder, R., Dai, Y., Maricic, I. & Kumar, V. Oligoclonality and innate-like features in the TCR repertoire of type II NKT cells reactive to a beta-linked self-glycolipid. Proc. Natl. Acad. Sci. USA 107, 10984–10989 (2010).
Gumperz, J.E. et al. Murine CD1d-restricted T cell recognition of cellular lipids. Immunity 12, 211–221 (2000).
Cox, D. et al. Determination of cellular lipids bound to human CD1d molecules. PLoS ONE 4, e5325 (2009).
Fox, L.M. et al. Recognition of lyso-phospholipids by human natural killer T lymphocytes. PLoS Biol. 7, e1000228 (2009).
Ni, Z., Okeley, N.M., Smart, B.P. & Gelb, M.H. Intracellular actions of group IIA secreted phospholipase A2 and group IVA cytosolic phospholipase A2 contribute to arachidonic acid release and prostaglandin production in rat gastric mucosal cells and transfected human embryonic kidney cells. J. Biol. Chem. 281, 16245–16255 (2006).
Ito, M. et al. Distribution of type V secretory phospholipase A2 expression in human hepatocytes damaged by liver disease. J. Gastroenterol. Hepatol. 19, 1140–1149 (2004).
Masuda, S., Murakami, M., Ishikawa, Y., Ishii, T. & Kudo, I. Diverse cellular localizations of secretory phospholipase A2 enzymes in several human tissues. Biochim. Biophys. Acta 1736, 200–210 (2005).
Kuwata, H., Yamamoto, S., Takekura, A., Murakami, M. & Kudo, I. Group IIA secretory phospholipase A2 is a unique 12/15-lipoxygenase–regulated gene in cytokine-stimulated rat fibroblastic 3Y1 cells. Biochim. Biophys. Acta 1686, 15–23 (2004).
Tischfield, J.A. et al. Low-molecular-weight, calcium-dependent phospholipase A2 genes are linked and map to homologous chromosome regions in mouse and human. Genomics 32, 328–333 (1996).
Brigl, M., Bry, L., Kent, S.C., Gumperz, J.E. & Brenner, M.B. Mechanism of CD1d-restricted natural killer T cell activation during microbial infection. Nat. Immunol. 4, 1230–1237 (2003).
Brigl, M. et al. Innate and cytokine-driven signals, rather than microbial antigens, dominate in natural killer T cell activation during microbial infection. J. Exp. Med. 208, 1163–1177 (2011).
Nagarajan, N.A. & Kronenberg, M. Invariant NKT cells amplify the innate immune response to lipopolysaccharide. J. Immunol. 178, 2706–2713 (2007).
Fujii, S., Liu, K., Smith, C., Bonito, A.J. & Steinman, R.M. The linkage of innate to adaptive immunity via maturing dendritic cells in vivo requires CD40 ligation in addition to antigen presentation and CD80/86 costimulation. J. Exp. Med. 199, 1607–1618 (2004).
Fujii, S., Shimizu, K., Smith, C., Bonifaz, L. & Steinman, R.M. Activation of natural killer T cells by α-galactosylceramide rapidly induces the full maturation of dendritic cells in vivo and thereby acts as an adjuvant for combined CD4 and CD8 T cell immunity to a coadministered protein. J. Exp. Med. 198, 267–279 (2003).
Ishak, K. et al. Histological grading and staging of chronic hepatitis. J. Hepatol. 22, 696–699 (1995).
Smiley, S.T., Kaplan, M.H. & Grusby, M.J. Immunoglobulin E production in the absence of interleukin-4–secreting CD1-dependent cells. Science 275, 977–979 (1997).
Cui, J. et al. Requirement for Vα14 NKT cells in IL-12–mediated rejection of tumors. Science 278, 1623–1626 (1997).
Van Kaer, L., Ashton-Rickardt, P.G., Ploegh, H.L. & Tonegawa, S. TAP1 mutant mice are deficient in antigen presentation, surface class I molecules, and CD4–8+ T cells. Cell 71, 1205–1214 (1992).
Postic, C. et al. Dual roles for glucokinase in glucose homeostasis as determined by liver and pancreatic beta cell–specific gene knock-outs using Cre recombinase. J. Biol. Chem. 274, 305–315 (1999).
Raabe, M. et al. Analysis of the role of microsomal triglyceride transfer protein in the liver of tissue-specific knockout mice. J. Clin. Invest. 103, 1287–1298 (1999).
Mohrs, M., Shinkai, K., Mohrs, K. & Locksley, R.M. Analysis of type 2 immunity in vivo with a bicistronic IL-4 reporter. Immunity 15, 303–311 (2001).
Stetson, D.B. et al. Constitutive cytokine mRNAs mark natural killer (NK) and NK T cells poised for rapid effector function. J. Exp. Med. 198, 1069–1076 (2003).
Chisari, F.V. et al. Structural and pathological effects of synthesis of hepatitis B virus large envelope polypeptide in transgenic mice. Proc. Natl. Acad. Sci. USA 84, 6909–6913 (1987).
Scapa, E.F. et al. Regulation of energy substrate utilization and hepatic insulin sensitivity by phosphatidylcholine transfer protein/StarD2. FASEB J. 22, 2579–2590 (2008).
Ernster, L., Siekevitz, P. & Palade, G.E. Enzyme-structure relationships in the endoplasmic reticulum of rat liver: a morphological and biochemical study. J. Cell Biol. 15, 541–562 (1962).
Folch, J., Lees, M. & Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 226, 497–509 (1957).
Acknowledgements
This work was supported by US National Institutes of Health (NIH) grants DK51362, DK44319, DK53056, DK88199, the Harvard Digestive Diseases Center DK034854 (to R.S.B.); the Deutsche Forschungsgemeinschaft (Ze 814/1-1, Ze 814/4-1), a Marie Curie International Reintegration Grant within the 7th European Community Framework Programme (256363) and the Crohn's and Colitis Foundation of America (to S.Z.); the Crohn's and Colitis Foundation of America, Austrian Science Fund, and Max Kade Foundation (to A.K.); NIH AR048632, AI049313 and the Burroughs Wellcome Fund for Translational Research (to D.B.M.); DK46900 (to M.M.H.); the NIH Intramural Research Program (K.M., Z.H. and T.J.L.); NIH grants AI068090, DK026743 and the Burroughs Wellcome Fund (to J.L.B.); and the A.P. Gianinni Foundation (to J.P.). We thank D.E. Cohen, E. Scapa and S.K. Dougan for insightful discussions.
Author information
Authors and Affiliations
Contributions
S.Z. designed, performed and analyzed experiments and prepared the manuscript with R.S.B. and T.J.L.; K.M. and Z.H. generated adenoviruses and adenoviral mutants and contributed to Ad-HBV studies; L.S. and D.B.M. designed, performed and analyzed LC-MS experiments together with S.Z.; J.P. and J.L.B. designed, performed and analyzed studies with HBV-Env mice; A.K. generated H-Mttp−/− mice and contributed to their characterization; K.B. and C.R. performed histopathological analyses; M.M.H. and J.I. obtained purified MTP; E.B. performed PLA2 inhibitor and siRNA studies; R.G. obtained primary HBV isolates; A.A. and J.H. contributed to human hepatocyte studies; S.S. contributed to supervision of the studies; T.J.L. and R.S.B. supervised the studies.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Data, Supplementary Methods, Supplementary Figures 1–19 and Supplementary Table 1 (PDF 2946 kb)
Rights and permissions
About this article
Cite this article
Zeissig, S., Murata, K., Sweet, L. et al. Hepatitis B virus–induced lipid alterations contribute to natural killer T cell–dependent protective immunity. Nat Med 18, 1060–1068 (2012). https://doi.org/10.1038/nm.2811
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.2811
This article is cited by
-
Dynamic modulations of urinary sphingolipid and glycerophospholipid levels in COVID-19 and correlations with COVID-19-associated kidney injuries
Journal of Biomedical Science (2022)
-
Distinct CD1d docking strategies exhibited by diverse Type II NKT cell receptors
Nature Communications (2019)
-
Tissue-specific functions of invariant natural killer T cells
Nature Reviews Immunology (2018)
-
Natural killer T cells mediate inflammation in the bile ducts
Mucosal Immunology (2018)
-
NKT cells in liver diseases
Frontiers of Medicine (2018)