Abstract
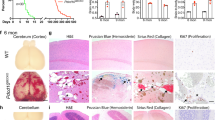
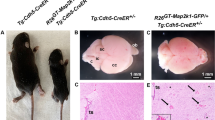
Cerebral cavernous malformations (CCMs) are human vascular malformations caused by mutations in three genes of unknown function: KRIT1, CCM2 and PDCD10. Here we show that the heart of glass (HEG1) receptor, which in zebrafish has been linked to ccm gene function, is selectively expressed in endothelial cells. Heg1−/− mice showed defective integrity of the heart, blood vessels and lymphatic vessels. Heg1−/−; Ccm2lacZ/+ and Ccm2lacZ/lacZ mice had more severe cardiovascular defects and died early in development owing to a failure of nascent endothelial cells to associate into patent vessels. This endothelial cell phenotype was shared by zebrafish embryos deficient in heg, krit1 or ccm2 and reproduced in CCM2-deficient human endothelial cells in vitro. Defects in the hearts of zebrafish lacking heg or ccm2, in the aortas of early mouse embryos lacking CCM2 and in the lymphatic vessels of neonatal mice lacking HEG1 were associated with abnormal endothelial cell junctions like those observed in human CCMs. Biochemical and cellular imaging analyses identified a cell-autonomous pathway in which the HEG1 receptor couples to KRIT1 at these cell junctions. This study identifies HEG1-CCM protein signaling as a crucial regulator of heart and vessel formation and integrity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Change history
27 January 2009
In the version of the supplementary information for this article originally posted online, the descriptions of Supplementary Videos 1–6 were incorrect. The errors have been corrected as of 27 January 2009.
12 February 2009
In this version of the article initially published, Shawn M. Sweeney was not included in the list of authors. The error has been corrected in the HTML and PDF versions of the article.
References
Rigamonti, D. et al. Cerebral cavernous malformations. Incidence and familial occurrence. N. Engl. J. Med. 319, 343–347 (1988).
Revencu, N. & Vikkula, M. Cerebral cavernous malformation: new molecular and clinical insights. J. Med. Genet. 43, 716–721 (2006).
Sahoo, T. et al. Mutations in the gene encoding KRIT1, a Krev-1/rap1a binding protein, cause cerebral cavernous malformations (CCM1). Hum. Mol. Genet. 8, 2325–2333 (1999).
Eerola, I. et al. KRIT1 is mutated in hyperkeratotic cutaneous capillary-venous malformation associated with cerebral capillary malformation. Hum. Mol. Genet. 9, 1351–1355 (2000).
Liquori, C.L. et al. Mutations in a gene encoding a novel protein containing a phosphotyrosine-binding domain cause type 2 cerebral cavernous malformations. Am. J. Hum. Genet. 73, 1459–1464 (2003).
Denier, C. et al. Mutations within the MGC4607 gene cause cerebral cavernous malformations. Am. J. Hum. Genet. 74, 326–337 (2004).
Bergametti, F. et al. Mutations within the programmed cell death 10 gene cause cerebral cavernous malformations. Am. J. Hum. Genet. 76, 42–51 (2005).
Zawistowski, J.S. et al. CCM1 and CCM2 protein interactions in cell signaling: implications for cerebral cavernous malformations pathogenesis. Hum. Mol. Genet. 14, 2521–2531 (2005).
Voss, K. et al. CCM3 interacts with CCM2 indicating common pathogenesis for cerebral cavernous malformations. Neurogenetics 8, 249–256 (2007).
Zhang, J., Rigamonti, D., Dietz, H.C. & Clatterbuck, R.E. Interaction between krit1 and malcavernin: implications for the pathogenesis of cerebral cavernous malformations. Neurosurgery 60, 353–359 (2007).
Mably, J.D., Mohideen, M.A., Burns, C.G., Chen, J.N. & Fishman, M.C. Heart of glass regulates the concentric growth of the heart in zebrafish. Curr. Biol. 13, 2138–2147 (2003).
Mably, J.D. et al. Santa and valentine pattern concentric growth of cardiac myocardium in the zebrafish. Development 133, 3139–3146 (2006).
Whitehead, K.J., Plummer, N.W., Adams, J.A., Marchuk, D.A. & Li, D.Y. Ccm1 is required for arterial morphogenesis: implications for the etiology of human cavernous malformations. Development 131, 1437–1448 (2004).
Plummer, N.W. et al. Neuronal expression of the Ccm2 gene in a new mouse model of cerebral cavernous malformations. Mamm. Genome 17, 119–128 (2006).
Wong, J.H., Awad, I.A. & Kim, J.H. Ultrastructural pathological features of cerebrovascular malformations: a preliminary report. Neurosurgery 46, 1454–1459 (2000).
Clatterbuck, R.E., Eberhart, C.G., Crain, B.J. & Rigamonti, D. Ultrastructural and immunocytochemical evidence that an incompetent blood-brain barrier is related to the pathophysiology of cavernous malformations. J. Neurol. Neurosurg. Psychiatry 71, 188–192 (2001).
Tu, J., Stoodley, M.A., Morgan, M.K. & Storer, K.P. Ultrastructural characteristics of hemorrhagic, nonhemorrhagic, and recurrent cavernous malformations. J. Neurosurg. 103, 903–909 (2005).
Glading, A., Han, J., Stockton, R.A. & Ginsberg, M.H. KRIT-1/CCM1 is a Rap1 effector that regulates endothelial cell cell junctions. J. Cell Biol. 179, 247–254 (2007).
Nakatsu, M.N. et al. Angiogenic sprouting and capillary lumen formation modeled by human umbilical vein endothelial cells (HUVEC) in fibrin gels: the role of fibroblasts and Angiopoietin-1. Microvasc. Res. 66, 102–112 (2003).
Folkman, J. & Haudenschild, C. Angiogenesis in vitro. Nature 288, 551–556 (1980).
Jin, S.W., Beis, D., Mitchell, T., Chen, J.N. & Stainier, D.Y. Cellular and molecular analyses of vascular tube and lumen formation in zebrafish. Development 132, 5199–5209 (2005).
Kamei, M. et al. Endothelial tubes assemble from intracellular vacuoles in vivo. Nature 442, 453–456 (2006).
Blum, Y. et al. Complex cell rearrangements during intersegmental vessel sprouting and vessel fusion in the zebrafish embryo. Dev. Biol. 316, 312–322 (2008).
Hogan, B.M., Bussmann, J., Wolburg, H. & Schulte-Merker, S. Ccm1 cell autonomously regulates endothelial cellular morphogenesis and vascular tubulogenesis in zebrafish. Hum. Mol. Genet. 17, 2424–2432 (2008).
Sebzda, E. et al. Syk and Slp-76 mutant mice reveal a cell-autonomous hematopoietic cell contribution to vascular development. Dev. Cell 11, 349–361 (2006).
Lawson, N.D. & Weinstein, B.M. In vivo imaging of embryonic vascular development using transgenic zebrafish. Dev. Biol. 248, 307–318 (2002).
Jin, S.W. et al. A transgene-assisted genetic screen identifies essential regulators of vascular development in vertebrate embryos. Dev. Biol. 307, 29–42 (2007).
Amsterdam, A. & Hopkins, N. Retroviral-mediated insertional mutagenesis in zebrafish. Methods Cell Biol. 77, 3–20 (2004).
Weinstein, B.M., Stemple, D.L., Driever, W. & Fishman, M.C. Gridlock, a localized heritable vascular patterning defect in the zebrafish. Nat. Med. 1, 1143–1147 (1995).
Lim, C.J. et al. Alpha4 integrins are type I cAMP-dependent protein kinase-anchoring proteins. Nat. Cell Biol. 9, 415–421 (2007).
Pfaff, M., Liu, S., Erle, D.J. & Ginsberg, M.H. Integrin beta cytoplasmic domains differentially bind to cytoskeletal proteins. J. Biol. Chem. 273, 6104–6109 (1998).
Acknowledgements
We thank C. Bertozzi, C. Chen, A. Granger, J. Lee, D. Li, P. Mericko, A. Schmaier, E. Sebzda, N. Shanbhag and K. Whitehead for valuable insights; M. Pack and J. He for assistance with zebrafish studies; R. Meade for preparation of the electron microscopy samples; and T. Branson for animal husbandry. This work was supported by the Swiss National Science Foundation, a Network of Excellence grant from the European Community (M.A.) and the US National Institutes of Health grants T32 HL07439 (to B.K.), T32 HL07971 (to X.Z.), HL078784 and AR27214 (to M.G.), HL62454 (to J.K.) and HL075380 and HL095326 (to M.L.K.).
Author information
Authors and Affiliations
Contributions
B.K. designed and performed all of the studies involving mutant mouse lines, contributed to endothelial cell culture and biochemical studies and wrote the manuscript; X.Z. designed and performed most of the zebrafish and biochemical studies; J.J.L., M.C., Z.Z., S.M.S. and L.G. contributed to the biochemical studies; Y.B. and M.C. contributed to the zebrafish studies; J.J.T. performed the endothelial cell culture studies; M.L. performed the immunohistochemistry studies; D.Z. contributed to the generation of mutant mouse lines; J.K. designed the endothelial cell culture studies and wrote the manuscript; M.A. designed some of the zebrafish studies and wrote the manuscript; M.H.G. designed some of the biochemical studies and wrote the manuscript; M.L.K. contributed to the design of mouse, zebrafish, endothelial and biochemical studies and wrote the manuscript.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figs. 1–12, Supplementary Tables 1 and 2, and Supplementary Methods (PDF 1210 kb)
Supplementary Movie 1
Intersegmental vessels of 24–26-h.p.f. fli1a:EGFP-cdc42 transgenic zebrafish embryos following injection of scrambled morpholino (control). The GFP-cdc42 fusion protein is expressed in endothelial cells and outlines the vacuoles that form and fuse during lumen formation. (AVI 4038 kb)
Supplementary Movie 2
Intersegmental vessels of 24–26-h.p.f. fli1a:EGFP-cdc42 transgenic zebrafish embryos following injection of morpholinos to block expression of heg. The GFP-cdc42 fusion protein is expressed in endothelial cells and outlines the vacuoles that form and fuse during lumen formation. Endothelial vacuole formation in zebrafish embryos lacking heg is similar to that observed in control embryos (Supplementary Movie 1). (AVI 1894 kb)
Supplementary Movie 3
Intersegmental vessels of 24–26-h.p.f. fli1a:EGFP-cdc42 transgenic zebrafish embryos following injection of morpholinos to block expression of ccm2. The GFP-cdc42 fusion protein is expressed in endothelial cells and outlines the vacuoles that form and fuse during lumen formation. Endothelial vacuole formation in zebrafish embryos lacking ccm2 is similar to that observed in control embryos (Supplementary Movie 1). (AVI 5678 kb)
Supplementary Movie 4
Red fluorescent quantum dots were injected into the dorsal aorta of 24–26-h.p.f. fli1a:EGFP-cdc42 transgenic zebrafish embryos following injection of scrambled morpholino (control). Patent intersegmental vessels are indicated by the presence of red fluorescent dots in the vessels. (AVI 2022 kb)
Supplementary Movie 5
Red fluorescent quantum dots were injected into the dorsal aorta of 24–26-h.p.f. fli1a:EGFP-cdc42 transgenic zebrafish embryos following injection of morpholinos to block expression of heg. The presence of red fluorescent dots in the intersegmental vessels indicates vessel patency. The presence of red fluorescent dots in the intersegmental vessels indicates that the intersegmental vessels of zebrafish embryos lacking heg are patent. (AVI 3405 kb)
Supplementary Movie 6
Red fluorescent quantum dots were injected into the dorsal aorta of 24–26-h.p.f. fli1a:EGFP-cdc42 transgenic zebrafish embryos following injection of morpholinos to block expression of ccm2. The presence of red fluorescent dots in the intersegmental vessels indicates vessel patency. The presence of red fluorescent dots in the intersegmental vessels indicates that the intersegmental vessels of zebrafish embryos lacking ccm2 are patent. (AVI 7446 kb)
Rights and permissions
About this article
Cite this article
Kleaveland, B., Zheng, X., Liu, J. et al. Regulation of cardiovascular development and integrity by the heart of glass–cerebral cavernous malformation protein pathway. Nat Med 15, 169–176 (2009). https://doi.org/10.1038/nm.1918
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.1918
This article is cited by
-
Genetics of brain arteriovenous malformations and cerebral cavernous malformations
Journal of Human Genetics (2023)
-
Epigenetics of type 2 diabetes and diabetes-related outcomes in the Strong Heart Study
Clinical Epigenetics (2022)
-
Myocardial protective effect and transcriptome profiling of Naoxintong on cardiomyopathy in zebrafish
Chinese Medicine (2021)
-
Novel Murine Models of Cerebral Cavernous Malformations
Angiogenesis (2020)
-
Cerebrovascular disorders associated with genetic lesions
Cellular and Molecular Life Sciences (2019)