Abstract
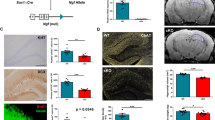
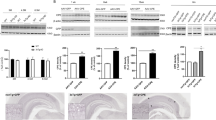
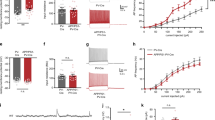
Profound neuronal dysfunction in the entorhinal cortex contributes to early loss of short-term memory in Alzheimer's disease1,2,3. Here we show broad neuroprotective effects of entorhinal brain-derived neurotrophic factor (BDNF) administration in several animal models of Alzheimer's disease, with extension of therapeutic benefits into the degenerating hippocampus. In amyloid-transgenic mice, BDNF gene delivery, when administered after disease onset, reverses synapse loss, partially normalizes aberrant gene expression, improves cell signaling and restores learning and memory. These outcomes occur independently of effects on amyloid plaque load. In aged rats, BDNF infusion reverses cognitive decline, improves age-related perturbations in gene expression and restores cell signaling. In adult rats and primates, BDNF prevents lesion-induced death of entorhinal cortical neurons. In aged primates, BDNF reverses neuronal atrophy and ameliorates age-related cognitive impairment. Collectively, these findings indicate that BDNF exerts substantial protective effects on crucial neuronal circuitry involved in Alzheimer's disease, acting through amyloid-independent mechanisms. BDNF therapeutic delivery merits exploration as a potential therapy for Alzheimer's disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
Change history
12 February 2009
In the version of this article initially published online, there was an error in the heat map in Figure 2h—the description ‘BDNF:aged’ applies only to the two rightmost columns, not the three rightmost columns. Additionally, some of the P values in the legends for Figure 1a, Figure 2b,f and Figure 3b,e were incorrect. Finally, ‘entorhinal’ was misspelled in Figure 1i. The errors have been corrected for the print, PDF and HTML versions of this article.
References
Gomez-Isla, T. et al. Profound loss of layer II entorhinal cortex neurons occurs in very mild Alzheimer's disease. J. Neurosci. 16, 4491–4500 (1996).
Kordower, J.H. et al. Loss and atrophy of layer II entorhinal cortex neurons in elderly people with mild cognitive impairment. Ann. Neurol. 49, 202–213 (2001).
Price, J.L. Neuron number in the entorhinal cortex and CA1 in preclinical Alzheimer disease. Arch. Neurol. 58, 1395–1402 (2001).
Squire, L.R. & Kandel, E.R. Memory Chapter 5 (Scientific American Library, New York, 2000).
Yan, Q. et al. Immunocytochemical localization of TrkB in the central nervous system of the adult rat. J. Comp. Neurol. 378, 135–137 (1997).
Kaplan, D.R. & Miller, F.D. Neurotrophin signal transduction in the nervous system. Curr. Opin. Neurobiol. 10, 381–391 (2000).
Narisawa-Saito, M., Wakabayashi, K., Tsuji, S., Takahashi, H. & Nawa, H. Regional specificity of alterations in NGF, BDNF and NT-3 levels in Alzheimer's disease. Neuroreport 7, 2925–2928 (1996).
Connor, B. et al. Brain-derived neurotrophic factor is reduced in Alzheimer's disease. Brain Res. Mol. Brain Res. 49, 71–81 (1997).
Hock, C., Heese, K., Hulette, C., Rosenberg, C. & Otten, U. Region-specific neurotrophin imbalances in Alzheimer disease: decreased levels of brain-derived neurotrophic factor and increased levels of nerve growth factor in hippocampus and cortical areas. Arch. Neurol. 57, 846–51 (2000).
Mucke, L. et al. High-level neuronal expression of aβ 1–42 in wild-type human amyloid protein precursor transgenic mice: synaptotoxicity without plaque formation. J. Neurosci. 20, 4050–4058 (2000).
Palop, J.J. et al. Neuronal depletion of calcium-dependent proteins in the dentate gyrus is tightly linked to Alzheimer's disease-related cognitive deficits. Proc. Natl. Acad. Sci. USA 100, 9572–9577 (2003).
Boddaert, J. et al. Evidence of a role for lactadherin in Alzheimer's disease. Am. J. Pathol. 170, 921–929 (2007).
Reddy, P.H. et al. Gene expression profiles of transcripts in amyloid precursor protein transgenic mice: up-regulation of mitochondrial metabolism and apoptotic genes is an early cellular change in Alzheimer's disease. Hum. Mol. Genet. 13, 1225–1240 (2004).
Scherzer, C.R. et al. Molecular markers of early Parkinson's disease based on gene expression in blood. Proc. Natl. Acad. Sci. USA 104, 955–960 (2007).
Morrison, J.H. & Hof, P.R. Life and death of neurons in the aging brain. Science 278, 412–419 (1997).
Merrill, D.A., Chiba, A.A. & Tuszynski, M.H. Conservation of neuronal number and size in the entorhinal cortex of behaviorally characterized aged rats. J. Comp. Neurol. 438, 445–456 (2001).
Merrill, D.A., Roberts, J.A. & Tuszynski, M.H. Conservation of neuron number and size in entorhinal cortex layers II, III and V/VI of aged primates. J. Comp. Neurol. 422, 396–401 (2000).
Rowe, W.B. et al. Hippocampal expression analyses reveal selective association of immediate-early, neuroenergetic and myelinogenic pathways with cognitive impairment in aged rats. J. Neurosci. 27, 3098–3110 (2007).
Holtzman, D.M. et al. TrkA expression in the CNS: evidence for the existence of several novel NGF-responsive CNS neurons. J. Neurosci. 15, 1567–1576 (1995).
Peterson, D.A., Lucidi-Phillipi, C.A., Eagle, K.L. & Gage, F.H. Perforant path damage results in progressive neuronal death and somal atrophy in layer II of entorhinal cortex and functional impairment with increasing postdamage age. J. Neurosci. 14, 6872–6885 (1994).
Rohn, T.T., Rissman, R.A., Head, E. & Cotman, C.W. Caspase activation in the Alzheimer's disease brain: tortuous and torturous. Drug News Perspect. 15, 549–557 (2002).
Tomac, A. et al. Protection and repair of the nigrostriatal dopaminergic system by GDNF in vivo. Nature 373, 335–339 (1995).
Winkler, C., Sauer, H., Lee, C.S. & Bjorklund, A. Short-term GDNF treatment provides long-term rescue of lesioned nigral dopaminergic neurons in a rat model of Parkinson's disease. J. Neurosci. 16, 7206–7215 (1996).
Gash, D.M. et al. Functional recovery in parkinsonian monkeys treated with GDNF. Nature 380, 252–255 (1996).
Kaspar, B.K., Llado, J., Sherkat, N., Rothstein, J.D. & Gage, F.H. Retrograde viral delivery of IGF-1 prolongs survival in a mouse ALS model. Science 301, 839–842 (2003).
Gazzaley, A.H., Thakker, M.M., Hof, P.R. & Morrison, J.H. Preserved number of entorhinal cortex layer II neurons in aged macaque monkeys. Neurobiol. Aging 18, 549–553 (1997).
Taffe, M.A., Weed, M.R., Gutierrez, T., Davis, S.A. & Gold, L.H. Modeling a task that is sensitive to dementia of the Alzheimer's type: individual differences in acquisition of a visuo-spatial paired-associate learning task in rhesus monkeys. Behav. Brain Res. 149, 123–133 (2004).
Gould, R.L., Brown, R.G., Owen, A.M., Fytche, D.H. & Howard, R.J. fMRI BOLD response to increasing task difficulty during successful paired associates learning. Neuroimage 20, 1006–1019 (2003).
Stoop, R. & Poo, M.M. Synaptic modulation by neurotrophic factors. Prog. Brain Res. 109, 359–364 (1996).
Levine, E.S., Crozier, R.A., Black, I.B. & Plummer, M.R. Brain-derived neurotrophic factor modulates hippocampal synaptic transmission by increasing N-methyl-d-aspartic acid receptor activity. Proc. Natl. Acad. Sci. USA 95, 10235–10239 (1998).
Figurov, A., Pozzo-Miller, L.D., Olafsson, P., Wang, T. & Lu, B. Regulation of synaptic responses to high-frequency stimulation and LTP by neurotrophins in the hippocampus. Nature 381, 706–709 (1996).
Thoenen, H. & Sendtmer, M. Neurotrophins: from enthusiastic expectations through sobering experiences to rational therapeutic approaches. Nat. Neurosci. 5, 1046–1050 (2002).
Kordower, J.H. et al. Clinicopathological findings following intraventricular glial-derived neurotrophic factor treatment in a patient with Parkinson's disease. Ann. Neurol. 46, 419–424 (1999).
Gill, S.S. et al. Direct brain infusion of glial cell line-derived neurotrophic factor in Parkinson disease. Nat. Med. 9, 589–595 (2003).
Tuszynski, M.H. et al. A phase 1 clinical trial of nerve growth factor gene therapy for Alzheimer disease. Nat. Med. 11, 551–555 (2005).
Marks, W.J., Jr. et al. Safety and tolerability of intraputaminal delivery of CERE-120 (adeno-associated virus serotype 2-neurturin) to patients with idiopathic Parkinson's disease: an open-label, phase I trial. Lancet Neurol. 7, 400–408 (2008).
Kaplitt, M.G. et al. Safety and tolerability of gene therapy with an adeno-associated virus (AAV) borne GAD gene for Parkinson's disease: an open label, phase I trial. Lancet 369, 2097–2105 (2007).
Smyth, G.K., Michaud, J. & Scott, H.S. Use of within-array replicate spots for assessing differential expression in microarray experiments. Bioinformatics 21, 2067–2075 (2005).
Owen, A.M., Roberts, A.C., Polkey, C.E., Sahakian, B.J. & Robbins, T.W. Extra-dimensional versus intra-dimensional set shifting performance following frontal lobe excisions, temporal lobe excisions or amygdalo-hippocampectomy in man. Neuropsychologia 29, 993–1006 (1991).
Acknowledgements
We thank C. Glabe (University of California, Irvine) for Aβ1–42 peptide. We thank D. Amaral and P. Lavenex for assistance with the primate perforant path model, K. Loew, T. Mead, R. Torres and M. Mateling for technical support, and F. Gao for data analysis. This work was supported by the US National Institutes of Health (AG10435), the California Regional Primate Research Center Base Grant, the Veterans Administration, the Alzheimer's Association, the State of California (04-35530), the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation and The Shiley Family Foundation.
Author information
Authors and Affiliations
Contributions
A.H.N. contributed surgery, data collection, analysis and manuscript writing for all studies; D.A.M. contributed data collection, analysis and manuscript writing for aging studies; G.C. and D.G. contributed to array studies and analysis; S.T. contributed to in vitro Aβ toxicity studies; B.E.S., G.M.S. and E.K. contributed to Aβ in vitro toxicity studies and breeding and behavior of APP mice; L.W. contributed stereology of APP mice; A.B. generated gene expression vectors; A.K. and M.V.C. contributed western blot analysis in aged rats; J.M.C. contributed ELISA and biochemical analyses; E.R. contributed surgery of APP mice; E.M. contributed synaptophysin quantification and surgery in APP mice; A.A.C. contributed surgery and behavioral analysis in aged rats and manuscript writing; M.H.T. contributed experimental design, surgery in rats and primates, data analysis and manuscript writing.
Corresponding author
Ethics declarations
Competing interests
M.H.T. is a scientific founder of Ceregene, Inc. and Trophin, Inc. Neither entity supported this research.
Supplementary information
Supplementary Text and Figures
Supplementary Figs. 1–4 and Supplementary Methods (PDF 647 kb)
Supplementary Data 1
Differentially expressed genes in APP-TG mice compared to WT, and effect of BDNF gene delivery (XLS 1038 kb)
Supplementary Data 2
Differentially expressed genes in aged rats compared to young, and effect of BDNF gene delivery to entorhinal cortex. (XLS 527 kb)
Rights and permissions
About this article
Cite this article
Nagahara, A., Merrill, D., Coppola, G. et al. Neuroprotective effects of brain-derived neurotrophic factor in rodent and primate models of Alzheimer's disease. Nat Med 15, 331–337 (2009). https://doi.org/10.1038/nm.1912
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.1912
This article is cited by
-
The probable role of tissue plasminogen activator/neuroserpin axis in Alzheimer’s disease: a new perspective
Acta Neurologica Belgica (2024)
-
Neuroprotection by Polyherbal Medicine Divya-Medha-Vati Against Scopolamine-Induced Cognitive Impairment Through Modulation of Oxidative Stress, Acetylcholine Activity, and Cell Signaling
Molecular Neurobiology (2024)
-
The therapeutic prospects and challenges of human neural stem cells for the treatment of Alzheimer's Disease
Cell Regeneration (2022)
-
Immune recognition of syngeneic, allogeneic and xenogeneic stromal cell transplants in healthy retinas
Stem Cell Research & Therapy (2022)
-
Clathrin-nanoparticles deliver BDNF to hippocampus and enhance neurogenesis, synaptogenesis and cognition in HIV/neuroAIDS mouse model
Communications Biology (2022)