Abstract
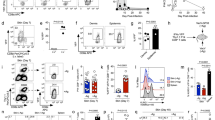
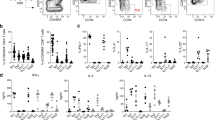
Lymphocytes travel throughout the body to carry out immune surveillance and participate in inflammatory reactions. Their path takes them from blood through tissues into lymph and back to blood. Molecules that control lymphocyte recruitment into extralymphoid tissues are well characterized, but exit is assumed to be random. Here, we showed that lymphocyte emigration from the skin was regulated and was sensitive to pertussis toxin. CD4+ lymphocytes emigrated more efficiently than CD8+ or B lymphocytes. T lymphocytes in the afferent lymph expressed functional chemokine receptor CCR7, and CCR7 was required for T lymphocyte exit from the skin. The regulated expression of CCR7 by tissue T lymphocytes may control their exit, acting with recruitment mechanisms to regulate lymphocyte transit and accumulation during immune surveillance and inflammation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Accession codes
References
Gowans, J.L. & Knight, E.J. The route of recirculation of lymphocytes in the rat. Proc. R. Soc. Lond. Ser. B. Biol. 159, 257–282 (1964).
Gowans, J.L. The recirculation of lymphocytes from blood to lymph in the rat. J. Physiol. (Lond.) 146, 54–69 (1959).
Mackay, C.R., Marston, W.L. & Dudler, L. Naive and memory T cells show distinct pathways of lymphocyte recirculation. J. Exp. Med. 171, 801–817 (1990).
Campbell, D.J., Debes, G.F., Johnston, B., Wilson, E. & Butcher, E.C. Targeting T cell responses by selective chemokine receptor expression. Semin. Immunol. 15, 277–286 (2003).
Olszewski, W.L., Grzelak, I., Ziolkowska, A. & Engeset, A. Immune cell traffic from blood through the normal human skin to lymphatics. Clin. Dermatol. 13, 473–483 (1995).
Olszewski, W.L. The lymphatic system in body homeostasis: physiological conditions. Lymphat. Res. Biol. 1, 11–21 (2003).
Klonowski, K.D. et al. Dynamics of blood-borne CD8 memory T cell migration in vivo. Immunity 20, 551–562 (2004).
Issekutz, T.B., Chin, W. & Hay, J.B. The characterization of lymphocytes migrating through chronically inflamed tissues. Immunology 46, 59–66 (1982).
Hall, J.G. & Morris, B. The origin of the cells in the efferent lymph from a single lymph node. J. Exp. Med. 121, 901–910 (1965).
Mackay, C.R., Kimpton, W.G., Brandon, M.R. & Cahill, R.N. Lymphocyte subsets show marked differences in their distribution between blood and the afferent and efferent lymph of peripheral lymph nodes. J. Exp. Med. 167, 1755–1765 (1988).
Young, A.J. The physiology of lymphocyte migration through the single lymph node in vivo. Semin. Immunol. 11, 73–83 (1999).
Seabrook, T. et al. The traffic of resting lymphocytes through delayed hypersensitivity and chronic inflammatory lesions: a dynamic equilibrium. Semin. Immunol. 11, 115–123 (1999).
Butcher, E.C. & Picker, L.J. Lymphocyte homing and homeostasis. Science 272, 60–66 (1996).
Mandala, S. et al. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science 296, 346–349 (2002).
Matloubian, M. et al. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature 427, 355–360 (2004).
Förster, R. et al. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell 99, 23–33 (1999).
Unsoeld, H., Krautwald, S., Voehringer, D., Kunzendorf, U. & Pircher, H. CCR7+ and CCR7− memory T cells do not differ in immediate effector cell function. J. Immunol. 169, 638–641 (2002).
Sallusto, F., Lenig, D., Förster, R., Lipp, M. & Lanzavecchia, A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401, 708–712 (1999).
Campbell, J.J. et al. CCR7 expression and memory T cell diversity in humans. J. Immunol. 166, 877–884 (2001).
Salmi, M., Koskinen, K., Henttinen, T., Elima, K. & Jalkanen, S. CLEVER-1 mediates lymphocyte transmigration through vascular and lymphatic endothelium. Blood 104, 3849–3857 (2004).
Abernethy, N.J., Hay, J.B., Kimpton, W.G., Washington, E.A. & Cahill, R.N. Non-random recirculation of small, CD4+ and CD8+ T lymphocytes in sheep: evidence for lymphocyte subset-specific lymphocyte-endothelial cell recognition. Int. Immunol. 2, 231–238 (1990).
Abernethy, N.J., Hay, J.B., Kimpton, W.G., Washington, E. & Cahill, R.N. Lymphocyte subset-specific and tissue-specific lymphocyte-endothelial cell recognition mechanisms independently direct the recirculation of lymphocytes from blood to lymph in sheep. Immunology 72, 239–245 (1991).
Sallusto, F., Geginat, J. & Lanzavecchia, A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu. Rev. Immunol. 22, 745–763 (2004).
Kim, C.H. et al. Rules of chemokine receptor association with T cell polarization in vivo. J. Clin. Invest. 108, 1331–1339 (2001).
Debes, G.F., Höpken, U.E. & Hamann, A. In vivo differentiated cytokine-producing CD4+ T cells express functional CCR7. J. Immunol. 168, 5441–5447 (2002).
Debes, G.F. et al. CC chemokine receptor 7 expression by effector/memory CD4+ T cells depends on antigen specificity and tissue localization during influenza A virus infection. J. Virol. 78, 7528–7535 (2004).
Gunn, M.D. et al. A chemokine expressed in lymphoid high endothelial venules promotes the adhesion and chemotaxis of naive T lymphocytes. Proc. Natl. Acad. Sci. USA 95, 258–263 (1998).
Saeki, H., Moore, A.M., Brown, M.J. & Hwang, S.T. Secondary lymphoid-tissue chemokine (SLC) and CC chemokine receptor 7 (CCR7) participate in the emigration pathway of mature dendritic cells from the skin to regional lymph nodes. J. Immunol. 162, 2472–2475 (1999).
Nakano, H. & Gunn, M.D. Gene duplications at the chemokine locus on mouse chromosome 4: multiple strain-specific haplotypes and the deletion of secondary lymphoid-organ chemokine and EBI-1 ligand chemokine genes in the plt mutation. J. Immunol. 166, 361–369 (2001).
Gunn, M.D. et al. Mice lacking expression of secondary lymphoid organ chemokine have defects in lymphocyte homing and dendritic cell localization. J. Exp. Med. 189, 451–460 (1999).
Ohl, L. et al. CCR7 governs skin dendritic cell migration under Inflammatory and steady-state conditions. Immunity 21, 279–288 (2004).
Martin-Fontecha, A. et al. Regulation of dendritic cell migration to the draining lymph node: impact on T lymphocyte traffic and priming. J. Exp. Med. 198, 615–621 (2003).
Eberhard, Y., Ortiz, S., Ruiz Lascano, A., Kuznitzky, R. & Serra, H.M. Up-regulation of the chemokine CCL21 in the skin of subjects exposed to irritants. BMC Immunol. 5, 7 (2004).
Stein, J.V. et al. The CC chemokine thymus-derived chemotactic agent 4 (TCA-4, secondary lymphoid tissue chemokine, 6Ckine, exodus-2) triggers lymphocyte function-associated antigen 1-mediated arrest of rolling T lymphocytes in peripheral lymph node high endothelial venules. J. Exp. Med. 191, 61–76 (2000).
Warnock, R.A. et al. The role of chemokines in the microenvironmental control of T versus B cell arrest in Peyer's patch high endothelial venules. J. Exp. Med. 191, 77–88 (2000).
Arnold, C.N., Butcher, E.C. & Campbell, D.J. Antigen-specific lymphocyte sequestration in lymphoid organs: lack of essential roles for αL and α4 integrin-dependent adhesion or Gαi protein-coupled receptor signaling. J. Immunol. 173, 866–873 (2004).
Reinhardt, R.L., Bullard, D.C., Weaver, C.T. & Jenkins, M.K. Preferential accumulation of antigen-specific effector CD4 T cells at an antigen injection site involves CD62E-dependent migration but not local proliferation. J. Exp. Med. 197, 751–762 (2003).
Young, A.J., Hein, W.R. & Hay, J.B. in Manual of Immunological Methods: the Comprehensive Source Book of Techniques (ed. Levkovits, I.) 2039–2059 (Academic Press, San Diego, 1997).
Vollmerhaus, B. in Lehrbuch der Anatomie der Haustiere III (eds Nickel, R., Schummer, A. & Seiferle, E.) 276–450 (Paul Parey, Berlin and Hamburg, 1984).
Hawley, R.G., Lieu, F.H., Fong, A.Z. & Hawley, T.S. Versatile retroviral vectors for potential use in gene therapy. Gene Ther. 1, 136–138 (1994).
Pear, W.S., Nolan, G.P., Scott, M.L. & Baltimore, D. Production of high-titer helper-free retroviruses by transient transfection. Proc. Natl. Acad. Sci. USA 90, 8392–8396 (1993).
Acknowledgements
We thank D. Campbell (University of Washington) for comments on the manuscript, and M. Zasio for sheep blood. Supported by the National Institutes of Health, the Department of Veterans Affairs (E.C.B.), the FACS Core of the Stanford Digestive Disease Center (National Institutes of Health P30 DK56339), Howard Hughes Medical Institute (C.N.A.), the Arthritis Foundation (G.F.D.) and Deutsche Forschungsgemeinschaft (G.F.D.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Debes, G., Arnold, C., Young, A. et al. Chemokine receptor CCR7 required for T lymphocyte exit from peripheral tissues. Nat Immunol 6, 889–894 (2005). https://doi.org/10.1038/ni1238
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni1238
This article is cited by
-
Tissue-resident memory T cells in the urogenital tract
Nature Reviews Nephrology (2022)
-
T helper cell trafficking in autoimmune kidney diseases
Cell and Tissue Research (2021)
-
Efficient homing of T cells via afferent lymphatics requires mechanical arrest and integrin-supported chemokine guidance
Nature Communications (2020)
-
Meningeal lymphatics clear erythrocytes that arise from subarachnoid hemorrhage
Nature Communications (2020)
-
T cells loaded with magnetic nanoparticles are retained in peripheral lymph nodes by the application of a magnetic field
Journal of Nanobiotechnology (2019)