Abstract
Obesity and resistance to insulin are closely associated with the development of low-grade inflammation. Interleukin 6 (IL-6) is linked to obesity-associated inflammation; however, its role in this context remains controversial. Here we found that mice with an inactivated gene encoding the IL-6Rα chain of the receptor for IL-6 in myeloid cells (Il6raΔmyel mice) developed exaggerated deterioration of glucose homeostasis during diet-induced obesity, due to enhanced resistance to insulin. Tissues targeted by insulin showed increased inflammation and a shift in macrophage polarization. IL-6 induced expression of the receptor for IL-4 and augmented the response to IL-4 in macrophages in a cell-autonomous manner. Il6raΔmyel mice were resistant to IL-4-mediated alternative polarization of macrophages and exhibited enhanced susceptibility to lipopolysaccharide (LPS)-induced endotoxemia. Our results identify signaling via IL-6 as an important determinant of the alternative activation of macrophages and assign an unexpected homeostatic role to IL-6 in limiting inflammation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Accession codes
References
Swinburn, B.A. et al. The global obesity pandemic: shaped by global drivers and local environments. Lancet 378, 804–814 (2011).
Xu, H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Invest. 112, 1821–1830 (2003).
Wellen, K.E. Obesity-induced inflammatory changes in adipose tissue. J. Clin. Invest. 112, 1785–1788 (2003).
Lumeng, C.N. & Saltiel, A.R. Inflammatory links between obesity and metabolic disease. J. Clin. Invest. 121, 2111–2117 (2011).
Arkan, M.C. et al. IKK-β links inflammation to obesity-induced insulin resistance. Nat. Med. 11, 191–198 (2005).
Cai, D. et al. Local and systemic insulin resistance resulting from hepatic activation of IKK-β and NF-κB. Nat. Med. 11, 183–190 (2005).
Kleinridders, A. et al. MyD88 signaling in the CNS is required for development of fatty acid-induced leptin resistance and diet-induced obesity. Cell Metab. 10, 249–259 (2009).
Donath, M.Y. & Shoelson, S.E. Type 2 diabetes as an inflammatory disease. Nat. Rev. Immunol. 11, 98–107 (2011).
Kahn, S.E., Hull, R.L. & Utzschneider, K.M. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 444, 840–846 (2006).
Pedersen, B.K. & Febbraio, M.A. Point: Interleukin-6 does have a beneficial role in insulin sensitivity and glucose homeostasis. J. Appl. Physiol. 102, 814–816 (2007).
Weiss, R. et al. Obesity and the metabolic syndrome in children and adolescents. N. Engl. J. Med. 350, 2362–2374 (2004).
Kim, H.-J. et al. Differential effects of interleukin-6 and -10 on skeletal muscle and liver insulin action in vivo. Diabetes 53, 1060–1067 (2004).
Wallenius, V. et al. Interleukin-6-deficient mice develop mature-onset obesity. Nat. Med. 8, 75–79 (2002).
Wunderlich, F.T. et al. Interleukin-6 signaling in liver-parenchymal cells suppresses hepatic inflammation and improves systemic insulin action. Cell Metab. 12, 237–249 (2010).
Clausen, B.E., Burkhardt, C., Reith, W., Renkawitz, R. & Förster, I. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res. 8, 265–277 (1999).
Vogt, M.C. & Brüning, J.C. CNS insulin signaling in the control of energy homeostasis and glucose metabolism—from embryo to old age. Trends Endocrinol. Metab. 24, 76–84 (2013).
Matthews, V.B. et al. Interleukin-6-deficient mice develop hepatic inflammation and systemic insulin resistance. Diabetologia 53, 2431–2441 (2010).
Belgardt, B.F. et al. Hypothalamic and pituitary c-Jun N-terminal kinase 1 signaling coordinately regulates glucose metabolism. Proc. Natl. Acad. Sci. USA 107, 6028–6033 (2010).
Hutchins, A.P., Poulain, S. & Miranda-Saavedra, D. Genome-wide analysis of STAT3 binding in vivo predicts effectors of the anti-inflammatory response in macrophages. Blood 119, e110–e119 (2012).
Portales-Casamar, E. et al. JASPAR 2010: the greatly expanded open-access database of transcription factor binding profiles. Nucleic Acids Res. 38, D105–D110 (2010).
Baumgartl, J. et al. Myeloid lineage cell-restricted insulin resistance protects apolipoproteinE-deficient mice against atherosclerosis. Cell Metab. 3, 247–256 (2006).
Mosser, D.M. & Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 8, 958–969 (2008).
Ricardo-Gonzalez, R.R. et al. IL-4/STAT6 immune axis regulates peripheral nutrient metabolism and insulin sensitivity. Proc. Natl. Acad. Sci. USA 107, 22617–22622 (2010).
Hirosumi, J. et al. A central role for JNK in obesity and insulin resistance. Nature 420, 333–336 (2002).
Lumeng, C.N., Bodzin, J.L. & Saltiel, A.R. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Invest. 117, 175–184 (2007).
Patsouris, D. et al. Ablation of CD11c-positive cells normalizes insulin sensitivity in obese insulin resistant animals. Cell Metab. 8, 301–309 (2008).
Odegaard, J.I. et al. Macrophage-specific PPARγ controls alternative activation and improves insulin resistance. Nature 447, 1116–1120 (2007).
Ouchi, N., Parker, J.L., Lugus, J.J. & Walsh, K. Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 11, 85–97 (2011).
Scheller, J., Chalaris, A., Schmidt-Arras, D. & Rose-John, S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim. Biophys. Acta 1813, 878–888 (2011).
Sadagurski, M. et al. Human IL6 enhances leptin action in mice. Diabetologia 53, 525–535 (2009).
Ellingsgaard, H. et al. Interleukin-6 enhances insulin secretion by increasing glucagon-like peptide-1 secretion from L cells and alpha cells. Nat. Med. 17, 1481–1489 (2011).
Herbert, D.R. et al. Alternative macrophage activation is essential for survival during schistosomiasis and downmodulates T helper 1 responses and immunopathology. Immunity 20, 623–635 (2004).
Vats, D. et al. Oxidative metabolism and PGC-1beta attenuate macrophage-mediated inflammation. Cell Metab. 4, 13–24 (2006).
Wermeling, F., Anthony, R.M., Brombacher, F. & Ravetch, J.V. Acute inflammation primes myeloid effector cells for anti-inflammatory STAT6 signaling. Proc. Nat. Acad. Sci. 110, 13487–13491 (2013).
Duluc, D. et al. Tumor-associated leukemia inhibitory factor and IL-6 skew monocyte differentiation into tumor-associated macrophage-like cells. Blood 110, 4319–4330 (2007).
Xing, Z. et al. IL-6 is an antiinflammatory cytokine required for controlling local or systemic acute inflammatory responses. J. Clin. Invest. 101, 311–320 (1998).
Spence, S. et al. Suppressors of cytokine signaling 2 and 3 diametrically control macrophage polarization. Immunity 38, 66–78 (2013).
Edgar, R., Domrachev, M. & Lash, A.E. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 30, 207–210 (2002).
Wunderlich, C.M. et al. Cutting edge: Inhibition of IL-6 trans-signaling protects from malaria-induced lethality in mice. J. Immunol. 188, 4141–4144 (2012).
Mauer, J. et al. Myeloid cell-restricted insulin receptor deficiency protects against obesity-induced inflammation and systemic insulin resistance. PLoS Genet. 6, e1000938 (2010).
Jordan, S.D. et al. Obesity-induced overexpression of miRNA-143 inhibits insulin-stimulated AKT activation and impairs glucose metabolism. Nat. Cell Biol. 13, 434–446 (2011).
Könner, A.C. et al. Insulin action in AgRP-expressing neurons is required for suppression of hepatic glucose production. Cell Metab. 5, 438–449 (2007).
Ferré, P., Leturque, A., Burnol, A.F., Pénicaud, L. & Girard, J. A method to quantify glucose utilization in vivo in skeletal muscle and white adipose tissue of the anaesthetized rat. Biochem. J. 228, 103–110 (1985).
Chaurasia, B. et al. Phosphoinositide-dependent kinase 1 provides negative feedback inhibition to Toll-like receptor-mediated NF-κB activation in macrophages. Mol. Cell. Biol. 30, 4354–4366 (2010).
Ruud, J. et al. Inflammation- and tumor-induced anorexia and weight loss require MyD88 in hematopoietic/myeloid cells but not in brain endothelial or neural cells. FASEB J. 27, 1973–1980 (2013).
Norris, A.W. et al. Muscle-specific PPARγ-deficient mice develop increased adiposity and insulin resistance but respond to thiazolidinediones. J. Clin. Invest. 112, 608–618 (2003).
Nguyen, K.D. et al. Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature 480, 104–108 (2011).
Schneider, C.A., Rasband, W.S. & Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675 (2012).
Günschmann, C. et al. Insulin/IGF-1 controls epidermal morphogenesis via regulation of FoxO-mediated p63 inhibition. Dev. Cell 26, 176–187 (2013).
Acknowledgements
We thank G. Schmall and T. Rayle for secretarial assistance, and B. Hampel and D. Kutyniok for technical assistance. Supported by the Deutsche Forschungsgemeinschaft (SFB 612 and SFB 670 to J.C.B.), the Leibniz Preis (BR 1492/7-1 to J.C.B.), the Cologne Excellence Cluster on Cellular Stress Responses in Aging Associated Diseases (funded by the Deutsche Forschungsgemeinschaft within the Excellence Initiative by German Federal and State Governments), the US National Institutes of Health (DP1AR064158, HL076746 and DK094641 to A.C.) and the National Health and Medical Research Council of Australia (APP1041760, APP1042465 and SPRF APP1021168 to M.A.F.).
Author information
Authors and Affiliations
Contributions
J.M., J.R., A.C., F.T.W. and J.C.B. designed the experiments; J.M., B.C., J.G., M.C.V., J.R. and K.D.N. did the experiments; S.T. helped with the flow cytometry; A.C.H. did the clamp operations; J.S. assisted during the ChIP analyses; H.S.B. did the indirect calorimetry; E.E., T.L.A., A.M., L.P. and M.A.F. supplied reagents; and J.M. and J.C.B. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
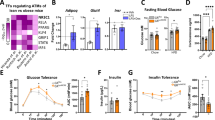
Supplementary Figure 1 Physiological characterization of Il6raΔmyel mice fed a NCD or HFD.
(a) Immunoblot of bone marrow-derived macrophages (BMDMs) generated from Il6rafl/fl mice (Ctrl) or Il6raΔmyel mice (Il6ra−/−) that were stimulated with IL-6 (50 ng/ml) for the indicated time points (Blot is representative of three independent experiments). (b) body composition (n=6), (c) fat pad weight (n=10), (d) serum leptin concentration (n=8), (e) oxygen (O2) consumption (n=6) and (f) daily caloric intake (n=8) of normal chow diet (NCD) or high fat diet (HFD) Il6rafl/fl and Il6raΔmyel mice. (Values are expressed as mean ±sem)
Supplementary Figure 2 Metabolic characterization of Il6raΔmyel mice fed a NCD or HFD.
NCD Il6rafl/fl or Il6raΔmyel mice were subjected to (a) glucose tolerance tests (GTT; n=12 vs 14) or (b) insulin tolerance tests (ITT; n=8 vs 14). (c) Blood glucose levels during euglycemic-hyperinsulinemic clamp analyses of HFD-fed Il6rafl/fl or Il6raΔmyel mice (n=8 vs 7). (d) Glucose infusion rate (GIR) during euglycemic-hyperinsulinemic clamp analyses (n=8 Il6rafl/fl; n=7 Il6raΔmyel; *p≤0.05; 2-Way-ANOVA with Bonferroni's post-test). (e) qRT-PCR analyses of livers from HFD Il6rafl/fl and Il6raΔmyel mice that were fasted for 16 hours (n=9 *p≤0.05; unpaired student's t-test; Data is expressed as % of Il6rafl/fl). (f) Triglyceride content in livers of HFD Il6rafl/fl and Il6raΔmyel mice that were fasted for 6 hours (n=11 vs 10; *p≤0.05; unpaired student's t-test). (g) Triglyceride concentration in serum of HFD Il6rafl/fl and Il6raΔmyel mice that were fasted for 6 hours (n=11 vs 10; p=0.08; unpaired student's t-test). (Values are expressed as mean ±sem)
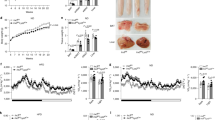
Supplementary Figure 3 Gene-expression profiles in the WAT, BAT and liver of Il6raΔmyel mice fed a NCD or HFD.
(a) Immunohistochemical staining of MAC2-positive cells in WAT from HFD Il6rafl/fl or Il6raΔmyel mice and quantification of MAC2-positive crown-like structures (CLS) in WAT from HFD-fed Il6rafl/fl or Il6raΔmyel mice (n=6 per genotype; *p≤0.05; unpaired student's t-test; Data is expressed as % CLS of adipocytes). (b) qRT-PCR analyses of WAT from NCD and HFD Il6rafl/fl or Il6raΔmyel mice (n=8 vs 8 NCD; n=7 vs 7 HFD; *p≤0.05, **p≤0.01, ***p≤0.001; 2-Way-ANOVA with Bonferroni's post-test; Data is expressed as % of NCD Il6rafl/fl). (c) qRT-PCR analyses of BAT from NCD and HFD Il6rafl/fl or Il6raΔmyel mice (n=4 vs 5 NCD; n=9 vs 9 HFD; *p≤0.05, **p≤0.01 vs NCD; 2-Way-ANOVA with Bonferroni's post-test; Data is expressed as % of NCD Il6rafl/fl). (d) qRT-PCR analyses of liver from NCD and HFD Il6rafl/fl or Il6raΔmyel mice (n=8 vs 8 NCD; n=9 vs 9 HFD; *p≤0.05, **p≤0.01, ***p≤0.001; 2-Way-ANOVA with Bonferroni's post-test; Data is expressed as % of NCD Il6rafl/fl). (Values are expressed as mean ±sem)
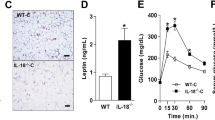
Supplementary Figure 4 Gene-expression profiles in metabolic tissues of Il6−/− and LysM-CreTg/wt mice fed a HFD and macrophage-autonomous effects of IL-6.
qRT-PCR analyses of (a) WAT and (b) liver from HFD wildtype (WT) and IL-6 knockout (Il6−/−) mice (n=7 vs 7 *p≤0.05, **p≤0.01; unpaired student's t-test; Data is expressed as % of WT). qRT- PCR analyses of (c) WAT, (d) BAT and (e) liver from HFD-fed wildtype (WT) and heterozygous LysM-Cre (LysM-CreTg/wt) mice (n=5vs5; Data is expressed as % of WT). (f) Representative Gene ontology analyses of the 15 highest scoring canonical pathways containing gene sets that were differentially expressed between Il6rafl/fl (Ctrl) and Il6ra−/− bone marrow-derived macrophages (BMDMS) after stimulation with IL-6 (50 ng/ml; 4 hours; Threshold 0.05; Fisher's Exact t-test). (g) qRT-PCR analyses of in Ctrl BMDMS that were left untreated or stimulated with IL-6 (50 ng/ml; 4 hours; Representative data from three independent experiments, each in triplicates; ***p≤0.001; unpaired student's t-test; Data is expressed as % of NT). (h) Representative FACS plots of IL-4Rα expression in wild-type BMDMS after treatment with IL-6. (i) qRT-PCR analyses of Ctrl or Il6ra−/− BMDMS that were left untreated or stimulated with IL-6 (50 ng/ml; 12 hours) (Representative data from three independent experiments, each in duplicates; *p≤0.01 vs Ctrl; **p≤0.001 vs NT; 2-Way-ANOVA with Bonferroni's post-test; Data is expressed as % of NT Ctrl). (j) Immunoblot of wild-type BMDMS that were left untreated (NT) or stimulated with IL-10 (10 ng/ml; 30 min) in the absence (IgG) or presence of an IL-10-neutralizing antibody (αIL-10) (n=3). (k) qRT-PCR analyses of siRNA-transfected wild-type BMDMS that were left untreated (NT) or IL-6-stimulated (4h, 50ng/ml) (n=3 independent experiments each in triplicates; *p≤0.001; 2-Way-ANOVA with Bonferroni's post-test; Data is expressed as % of NT Ctrl siRNA). (Values are expressed as mean ±sem)
Supplementary Figure 5 STAT3-binding site prediction and ChIP analyses of IL-6-stimulated macophages.
(a) JASPAR prediction analysis of putative STAT3-binding sites in the Il4ra and Socs3 promoter (b) ChIP qRT-PCR showing occupancy of p-STAT3 over the Socs3 promoter (left panel) and over a non-open reading frame region (negative control IGX1A; right panel) in Il6rafl/fl BMDMs (Ctrl) and Il6ra−/− BMDMS stimulated with IL-6 (50ng/ml) for the indicated time points (n=3 vs 3 independent experiments; *p≤0.001 vs Ctrl **p≤0.001 vs NT; 2-Way-ANOVA with Bonferroni's post-test; Data is expressed as % of NT Ctrl). (c) qRT-PCR Cycle threshold (Ct) values obtained with the indicated primer sets on DNA samples from IgG ChIP (n=3 vs 3 independent experiments). (Values are expressed as mean ±sem)
Supplementary Figure 6 Effects of IL-6 and IL-4 on macrophages in vitro and in vivo.
(a) qRT-PCR analyses of bone marrow-derived macrophages (BMDMS) from Il6rafl/fl (Ctrl) or Il6raΔmyel (Il6ra−/−) mice that were left untreated or stimulated with IL-6 (50 ng/ml; 12 hours) (n=6; *p≤0.01 vs Ctrl **p≤0.001 vs NT; 2-Way-ANOVA with Bonferroni's post-test; Data is expressed as % of NT Ctrl). (b) Representative FACS plots of expression of CD206 and ARG1 in wild-type BMDMS. (c) qRT-PCR analyses of Ctrl BMDMS and Il6ra−/− BMDM that were left untreated or stimulated with IL-6 (50 ng/ml; 12 hours) and subsequently exposed to IL-4 (10 ng/ml) alone or IL-4 in combination with IL-6 for an additional 24 hours (n=6; *p≤0.05 vs Ctrl **p≤0.01 vs IL-4; 2-Way-ANOVA with Bonferroni's post-test; data is expressed as % of IL-4 Ctrl). (d) Gating strategy for FACS analysis of adipose tissue, blood, and peritoneum (e) Representative FACS plots of expression of CD206 in WAT, BAT, blood and peritoneal cavity of wild-type mice. (Values are expressed as mean ±sem)
Supplementary Figure 7 Effects of IL-4 treatment in Il6raΔmyel mice fed a HFD and proposed model.
(a) Glucose tolerance tests (GTT) of HFD Il6rafl/fl (left panel) and HFD Il6raΔmyel mice (right panel) before (basal) and after a 4-week treatment period with IL-4 (n=15 vs 18). (b) (left panel) Homeostatic model assessment of insulin resistance (HOMA-IR) indices of HFD Il6rafl/fl and HFD Il6raΔmyel mice before (basal) and after a 4-week treatment with IL-4 (basal n=8 vs 7; IL-4 n=15 vs 18; *p≤0.01; 2-Way-ANOVA with Bonferroni's post-test). (right panel) Percentual improvement of HOMA-IR indices upon IL-4 treatment (n=8vs7; *p≤0.05; unpaired student's t-test; Data is expressed as % of basal). (Values are expressed as mean ± sem). (c) Proposed model: Pro-inflammatory conditions such as obesity or endotoxemia lead to increased serum concentrations of free fatty acids (FFA), bacterial lipopolysaccharides (LPS) and, among other cytokines, interleukin 6 (IL-6). FFA and LPS on one hand stimulate toll-like receptor 4 (TLR4) to activate expression of pro-inflammatory mediators such as TNFα, IL1β, IL-12 and iNOS, which are associated with classical M1 macrophage activation. IL-6 on the other hand activates STAT3 to induce expression of the IL-4 receptor. The increased abundance of IL-4 receptors on the cell surface leads to enhanced sensitivity to IL-4, which is thought to mainly stem from eosinophils and CD4+ T-cells. Binding of IL-4 to its receptor activates anti-inflammatory STAT6, which is a central transcriptional activator of factors related to alternative M2 macrophage activation, such as MRC1, ARG1, Retnla/FIZZ1 and IL-10. IL-6- and IL-4-dependent signaling cascades then act synergistically to inhibit expression of M1-associated genes and to activate M2-associated genes, ultimately tilting the balance towards increased numbers of M2 macrophages. This shift in macrophage polarization by combined IL-6- and IL-4-action finally serves to limit inflammation, to retain insulin sensitivity and to restore homeostasis during sepsis.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–7 and Supplementary Table 1 (PDF 6606 kb)
Rights and permissions
About this article
Cite this article
Mauer, J., Chaurasia, B., Goldau, J. et al. Signaling by IL-6 promotes alternative activation of macrophages to limit endotoxemia and obesity-associated resistance to insulin. Nat Immunol 15, 423–430 (2014). https://doi.org/10.1038/ni.2865
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.2865
This article is cited by
-
Resveratrol-βcd inhibited premature ovarian insufficiency progression by regulating granulosa cell autophagy
Journal of Ovarian Research (2024)
-
Muscle-to-organ cross-talk mediated by interleukin 6 during exercise: a review
Sport Sciences for Health (2024)
-
MSCs’ conditioned media cytokine and growth factor profiles and their impact on macrophage polarization
Stem Cell Research & Therapy (2023)
-
Exosomes derived from LPS-preconditioned bone marrow-derived MSC modulate macrophage plasticity to promote allograft survival via the NF-κB/NLRP3 signaling pathway
Journal of Nanobiotechnology (2023)
-
IL-6/ERK signaling pathway participates in type I IFN-programmed, unconventional M2-like macrophage polarization
Scientific Reports (2023)