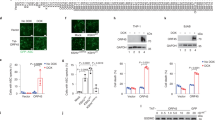
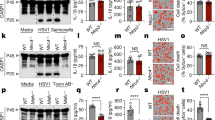
Abstract
Interleukin 1β (IL-1β) is a potent proinflammatory factor during viral infection. Its production is tightly controlled by transcription of Il1b dependent on the transcription factor NF-κB and subsequent processing of pro-IL-1β by an inflammasome. However, the sensors and mechanisms that facilitate RNA virus–induced production of IL-1β are not well defined. Here we report a dual role for the RNA helicase RIG-I in RNA virus–induced proinflammatory responses. Whereas RIG-I-mediated activation of NF-κB required the signaling adaptor MAVS and a complex of the adaptors CARD9 and Bcl-10, RIG-I also bound to the adaptor ASC to trigger caspase-1-dependent inflammasome activation by a mechanism independent of MAVS, CARD9 and the Nod-like receptor protein NLRP3. Our results identify the CARD9–Bcl-10 module as an essential component of the RIG-I-dependent proinflammatory response and establish RIG-I as a sensor able to activate the inflammasome in response to certain RNA viruses.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Change history
15 May 2013
In 2010 we reported in Nature Immunology how the sensing of cytosolic RNA triggers the generation of mature interleukin 1β (IL-1β; Poeck et al., Nat. Immunol. 11, 63–69 (2010)). We demonstrated that ligation of the RNA helicase RIG-I triggered the adaptor CARD9 downstream of the signaling adaptor MAVS (IPS-1) to induce the transcription factor NF-κB for the generation of pro-IL-1β and in parallel activated caspase-1 for the generation of mature IL-1β. We presented data that indicated similar generation of pro-IL-1β and IL-1β in wild-type bone marrow–derived dendritic cells (BMDCs) and BMDCs deficient in CARD9 or the adaptor Bcl-10 after transfection of the synthetic DNA poly(dA:dT) (Fig. 3c (right) and Fig. 4c,e,f). However, additional experiments in our laboratory have shown that CARD9- or Bcl-10-deficient BMDCs have impaired production of pro-IL-1β and IL-1β not only after sensing of RNA but also after the transfection of poly(dA:dT). We speculate that a particular aliquot of poly(dA:dT) used during the preparation of our study published in 2010 might have been contaminated with lipopolysaccharide or some other CARD9-independent trigger of the innate immune system. Still, the central conclusions of that manuscript (that RIG-I signals via CARD9 and the inflammasome to control IL-1β) remain unchanged and have been confirmed by multiple independent laboratories. Nevertheless, we would like to correct the idea that CARD9- or Bcl-10-deficient cells have normal IL-1β responses to the transfection of poly(dA:dT).
References
Kawai, T. & Akira, S. Toll-like receptor and RIG-I-like receptor signaling. Ann. NY Acad. Sci. 1143, 1–20 (2008).
Alexopoulou, L., Holt, A.C., Medzhitov, R. & Flavell, R.A. Recognition of double-stranded RNA and activation of NF-κB by Toll-like receptor 3. Nature 413, 732–738 (2001).
Heil, F. et al. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science 303, 1526–1529 (2004).
Hemmi, H. et al. Toll-like receptor recognizes bacterial DNA. Nature 408, 740–745 (2000).
Burckstummer, T. et al. An orthogonal proteomic-genomic screen identifies AIM2 as a cytoplasmic DNA sensor for the inflammasome. Nat. Immunol. 10, 266–272 (2009).
Fernandes-Alnemri, T., Yu, J.W., Datta, P., Wu, J. & Alnemri, E.S. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature 458, 509–513 (2009).
Hornung, V. et al. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature 458, 514–518 (2009).
Roberts, T.L. et al. HIN-200 proteins regulate caspase activation in response to foreign cytoplasmic DNA. Science 323, 1057–1060 (2009).
Andrejeva, J. et al. The V proteins of paramyxoviruses bind the IFN-inducible RNA helicase, mda-5, and inhibit its activation of the IFN-β promoter. Proc. Natl. Acad. Sci. USA 101, 17264–17269 (2004).
Yoneyama, M. et al. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat. Immunol. 5, 730–737 (2004).
Pichlmair, A. & Reis e Sousa, C. Innate recognition of viruses. Immunity 27, 370–383 (2007).
Hornung, V. et al. 5′-Triphosphate RNA is the ligand for RIG-I. Science 314, 994–997 (2006).
Pichlmair, A. et al. RIG-I-mediated antiviral responses to single-stranded RNA bearing 5′-phosphates. Science 314, 997–1001 (2006).
Schlee, M. et al. Recognition of 5′ triphosphate by RIG-I helicase requires short blunt double-stranded RNA as contained in panhandle of negative-strand virus. Immunity 31, 25–34 (2009).
Schmidt, A. et al. 5′-triphosphate RNA requires base-paired structures to activate antiviral signaling via RIG-I. Proc. Natl. Acad. Sci. USA 106, 12067–12072 (2009).
Kato, H. et al. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature 441, 101–105 (2006).
Kawai, T. et al. IPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon induction. Nat. Immunol. 6, 981–988 (2005).
Meylan, E. et al. Cardif is an adaptor protein in the RIG-I antiviral pathway and is targeted by hepatitis C virus. Nature 437, 1167–1172 (2005).
Seth, R.B., Sun, L., Ea, C.K. & Chen, Z.J. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-κB and IRF 3. Cell 122, 669–682 (2005).
Nakhaei, P., Genin, P., Civas, A. & Hiscott, J. RIG-I-like receptors: Sensing and responding to RNA virus infection. Semin. Immunol. 21, 215–222 (2009).
Yu, H.B. & Finlay, B.B. The caspase-1 inflammasome: a pilot of innate immune responses. Cell Host Microbe 4, 198–208 (2008).
Allen, I.C. et al. The NLRP3 inflammasome mediates in vivo innate immunity to influenza a virus through recognition of viral RNA. Immunity 30, 556–565 (2009).
Gross, O. et al. Syk kinase signalling couples to the Nlrp3 inflammasome for anti-fungal host defence. Nature 459, 433–436 (2009).
Ichinohe, T., Lee, H.K., Ogura, Y., Flavell, R. & Iwasaki, A. Inflammasome recognition of influenza virus is essential for adaptive immune responses. J. Exp. Med. 206, 79–87 (2009).
Thomas, P.G. et al. The intracellular sensor NLRP3 mediates key innate and healing responses to influenza A virus via the regulation of caspase-1. Immunity 30, 566–575 (2009).
Poeck, H. et al. 5′-Triphosphate-siRNA: turning gene silencing and Rig-I activation against melanoma. Nat. Med. 14, 1256–1263 (2008).
Gack, M.U. et al. TRIM25 RING-finger E3 ubiquitin ligase is essential for RIG-I-mediated antiviral activity. Nature 446, 916–920 (2007).
Hara, H. & Saito, T. CARD9 versus CARMA1 in innate and adaptive immunity. Trends Immunol. 30, 234–242 (2009).
Goodridge, H.S. et al. Differential use of CARD9 by dectin-1 in macrophages and dendritic cells. J. Immunol. 182, 1146–1154 (2009).
Gross, O. et al. Card9 controls a non-TLR signalling pathway for innate anti-fungal immunity. Nature 442, 651–656 (2006).
Wang, L. et al. Card10 is a novel caspase recruitment domain/membrane-associated guanylate kinase family member that interacts with BCL10 and activates NF-κB. J. Biol. Chem. 276, 21405–21409 (2001).
Rawlings, D.J., Sommer, K. & Moreno-Garcia, M.E. The CARMA1 signalosome links the signalling machinery of adaptive and innate immunity in lymphocytes. Nat. Rev. Immunol. 6, 799–812 (2006).
Henry, T., Brotcke, A., Weiss, D.S., Thompson, L.J. & Monack, D.M. Type I interferon signaling is required for activation of the inflammasome during Francisella infection. J. Exp. Med. 204, 987–994 (2007).
Steinberg, C. et al. The IFN regulatory factor 7-dependent type I IFN response is not essential for early resistance against murine cytomegalovirus infection. Eur. J. Immunol. 39, 1007–1018 (2009).
Schroder, K., Muruve, D.A. & Tschopp, J. Innate immunity: cytoplasmic DNA sensing by the AIM2 inflammasome. Curr. Biol. 19, R262–R265 (2009).
Colonna, M. All roads lead to CARD9. Nat. Immunol. 8, 554–555 (2007).
Geijtenbeek, T.B. & Gringhuis, S. I. Signalling through C-type lectin receptors: shaping immune responses. Nat. Rev. Immunol. 9, 465–479 (2009).
Werninghaus, K. et al. Adjuvanticity of a synthetic cord factor analogue for subunit Mycobacterium tuberculosis vaccination requires FcRgamma-Syk-Card9-dependent innate immune activation. J. Exp. Med. 206, 89–97 (2009).
Hsu, Y.M. et al. The adaptor protein CARD9 is required for innate immune responses to intracellular pathogens. Nat. Immunol. 8, 198–205 (2007).
Hara, H. et al. The adaptor protein CARD9 is essential for the activation of myeloid cells through ITAM-associated and Toll-like receptors. Nat. Immunol. 8, 619–629 (2007).
Su, H. et al. Requirement for caspase-8 in NF-κB activation by antigen receptor. Science 307, 1465–1468 (2005).
Takahashi, K. et al. Roles of caspase-8 and caspase-10 in innate immune responses to double-stranded RNA. J. Immunol. 176, 4520–4524 (2006).
Bauernfeind, F.G. et al. Cutting edge: NF-κB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J. Immunol. 183, 787–791 (2009).
Franchi, L., Eigenbrod, T. & Nunez, G. Cutting edge: TNF-α mediates sensitization to ATP and silica via the NLRP3 inflammasome in the absence of microbial stimulation. J. Immunol. 183, 792–796 (2009).
Dostert, C. et al. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science 320, 674–677 (2008).
Gitlin, L. et al. Essential role of mda-5 in type I IFN responses to polyriboinosinic:polyribocytidylic acid and encephalomyocarditis picornavirus. Proc. Natl. Acad. Sci. USA 103, 8459–8464 (2006).
Mariathasan, S. et al. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature 430, 213–218 (2004).
Michallet, M.C. et al. TRADD protein is an essential component of the RIG-like helicase antiviral pathway. Immunity 28, 651–661 (2008).
Petrilli, V. et al. Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Differ. 14, 1583–1589 (2007).
LeibundGut-Landmann, S. et al. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat. Immunol. 8, 630–638 (2007).
Acknowledgements
We thank J. Tschopp (University of Lausanne) for critical reading of the manuscript, discussions and NLRP3-deficient, ASC-deficient and MAVS-deficient mice and plasmids; and A. Krug (Technical University of Munich) for EMCV. This work includes parts of a thesis by M.B. at the University of Munich. Supported by Bundesministerium für Bildung und Forschung Biofuture (G.H.), Deutsche Forschungsgemeinschaft (SFB704, SFB670, SFB832 and KFO177 to G.H.; Sonderforschungsbereiche to S.E., V.H. and J.R.; Graduiertenkolleg 1202 to M.B.; and RO 2525/3–1 to S.R.), the Center for Integrated Protein Science Munich (S.E.), the European Research Council (V.H.) and Deutsche Krebshilfe (J.R.).
Author information
Authors and Affiliations
Contributions
H.P., M.B., O.G., G.H., V.H. and J.R. designed the research; H.P., M.B., O.G., K.F., S.R., N.H., M.R. and M.S. did experiments; W.B., H.K., S.A. and S.I. contributed critical reagents; H.P., M.B., O.G., S.R., S.E., C.P., V.H., G.H. and J.R. analyzed results; H.P. and M.B. prepared the figures; and H.P., M.B., O.G., G.H. and J.R. wrote the paper.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–4 and Supplementary Table 1 (PDF 936 kb)
Rights and permissions
About this article
Cite this article
Poeck, H., Bscheider, M., Gross, O. et al. Recognition of RNA virus by RIG-I results in activation of CARD9 and inflammasome signaling for interleukin 1β production. Nat Immunol 11, 63–69 (2010). https://doi.org/10.1038/ni.1824
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.1824
This article is cited by
-
Potassium ion channels as a molecular target to reduce virus infection and mortality of honey bee colonies
Virology Journal (2023)
-
Local immunotherapy with the RNA-based immune stimulator CV8102 induces substantial anti-tumor responses and enhances checkpoint inhibitor activity
Cancer Immunology, Immunotherapy (2023)
-
Card9 protects sepsis by regulating Ripk2-mediated activation of NLRP3 inflammasome in macrophages
Cell Death & Disease (2022)
-
EV-A71 induced IL-1β production in THP-1 macrophages is dependent on NLRP3, RIG-I, and TLR3
Scientific Reports (2022)
-
Insights into inflammasome regulation: cellular, molecular, and pathogenic control of inflammasome activation
Immunologic Research (2022)