Abstract
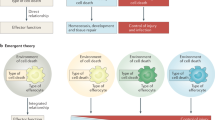
Death receptors (DRs) are members of the tumor necrosis factor receptor superfamily that possess a cytoplasmic death domain (DD). DRs regulate important operational and homeostatic aspects of the immune system. They transmit signals through apical protein complexes, which are nucleated by the DD adaptors FADD and TRADD, to control cellular outcomes that range from apoptosis to gene activation. FADD and TRADD also nucleate several distal signaling complexes, which mediate cross-talk between distinct DR signaling pathways. Moreover, together with other DR signal transducers, FADD and TRADD participate in functional complexes assembled by certain non-DR immune cell receptors, such as pattern-recognition receptors. Thus, DR signal transducers may provide important nodes of coordination in immune signaling networks.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Locksley, R.M., Killeen, N. & Lenardo, M.J. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell 104, 487–501 (2001).
Ashkenazi, A. & Dixit, V.M. Death receptors: signaling and modulation. Science 281, 1305–1308 (1998).
Wu, G.S., Burns, T.F., Zhan, Y., Alnemri, E.S. & El-Deiry, W.S. Molecular cloning and functional analysis of the mouse homologue of the KILLER/DR5 tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) death receptor. Cancer Res. 59, 2770–2775 (1999).
Festjens, N., Vanden Berghe, T., Cornelis, S. & Vandenabeele, P. RIP1, a kinase on the crossroads of a cell's decision to live or die. Cell Death Differ. 14, 400–410 (2007).
Varfolomeev, E. & Vucic, D. (Un)expected roles of c-IAPs in apoptotic and NFκB signaling pathways. Cell Cycle 7, 1511–1521 (2008).
Ashkenazi, A. Targeting death and decoy receptors of the tumour-necrosis factor superfamily. Nat. Rev. Cancer 2, 420–430 (2002).
Pan, G. et al. Identification and functional characterization of DR6, a novel death domain-containing TNF receptor. FEBS Lett. 431, 351–356 (1998).
Micheau, O. & Tschopp, J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell 114, 181–190 (2003).
Wang, L., Du, F. & Wang, X. TNF-α induces two distinct caspase-8 activation pathways. Cell 133, 693–703 (2008). Provides evidence that two distinct cytoplasmic complexes downstream of TNFR1 can activate caspase-8-mediated apoptosis; this is consistent with an earlier study (ref. 8).
Varfolomeev, E. et al. Molecular determinants of kinase pathway activation by Apo2 ligand/tumor necrosis factor-related apoptosis-inducing ligand. J. Biol. Chem. 280, 40599–40608 (2005). Describes a secondary complex downstream of DR5 that activates NF-κB, JNK and p38 MAPK.
Koenig, A., Russell, J.Q., Rodgers, W.A. & Budd, R.C. Spatial differences in active caspase-8 defines its role in T-cell activation versus cell death. Cell Death Differ. 15, 1701–1711 (2008).
Su, H. et al. Requirement for caspase-8 in NF-κB activation by antigen receptor. Science 307, 1465–1468 (2005).
Chun, H.J. et al. Pleiotropic defects in lymphocyte activation caused by caspase-8 mutations lead to human immunodeficiency. Nature 419, 395–399 (2002). Lymphocytes from humans with homozygous caspase-8 mutations are resistant to DR-induced apoptosis but also show defective proliferation, suggesting DR-independent caspase-8 functions.
Chen, N.J. et al. Beyond tumor necrosis factor receptor: TRADD signaling in toll-like receptors. Proc. Natl. Acad. Sci. USA 105, 12429–12434 (2008).
Pobezinskaya, Y.L. et al. The function of TRADD in signaling through tumor necrosis factor receptor 1 and TRIF-dependent Toll-like receptors. Nat. Immunol. 9, 1047–1054 (2008).
Ermolaeva, M.A. et al. Function of TRADD in tumor necrosis factor receptor 1 signaling and in TRIF-dependent inflammatory responses. Nat. Immunol. 9, 1037–1046 (2008). TRADD knockout studies (refs. 15 and 16 ) confirm the role of TRADD in TNFR1 signaling and further implicate TRADD in TRIF-mediated TLR pathways.
Ma, Y. et al. Fas ligation on macrophages enhances IL-1R1-Toll-like receptor 4 signaling and promotes chronic inflammation. Nat. Immunol. 5, 380–387 (2004).
Imtiyaz, H.Z. et al. The Fas-associated death domain protein is required in apoptosis and TLR-induced proliferative responses in B cells. J. Immunol. 176, 6852–6861 (2006).
Michallet, M.C. et al. TRADD protein is an essential component of the RIG-like helicase antiviral pathway. Immunity 28, 651–661 (2008). Identifies TRADD as an essential component not only for proinflammatory TNFR1 signaling but also for RLH signaling.
Takahashi, K. et al. Roles of caspase-8 and caspase-10 in innate immune responses to double-stranded RNA. J. Immunol. 176, 4520–4524 (2006). Implicates human caspases 8 and 10 as components of the RLH pathway that mediates NF-κB–dependent inflammatory responses.
Peter, M.E. & Krammer, P.H. The CD95(APO-1/Fas) DISC and beyond. Cell Death Differ. 10, 26–35 (2003).
Feig, C., Tchikov, V., Schutze, S. & Peter, M.E. Palmitoylation of CD95 facilitates formation of SDS-stable receptor aggregates that initiate apoptosis signaling. EMBO J. 26, 221–231 (2007).
Muppidi, J.R. & Siegel, R.M. Ligand-independent redistribution of Fas (CD95) into lipid rafts mediates clonotypic T cell death. Nat. Immunol. 5, 182–189 (2004).
Wagner, K.W. et al. Death-receptor O-glycosylation controls tumor-cell sensitivity to the proapoptotic ligand Apo2L/TRAIL. Nat. Med. 13, 1070–1077 (2007).
Sakamaki, K., Tsukumo, S. & Yonehara, S. Molecular cloning and characterization of mouse caspase-8. Eur. J. Biochem. 253, 399–405 (1998).
Krammer, P.H., Arnold, R. & Lavrik, I.N. Life and death in peripheral T cells. Nat. Rev. Immunol. 7, 532–542 (2007).
Budd, R.C., Yeh, W.C. & Tschopp, J. cFLIP regulation of lymphocyte activation and development. Nat. Rev. Immunol. 6, 196–204 (2006).
Ueffing, N. et al. Mutational analyses of c-FLIPR, the only murine short FLIP isoform, reveal requirements for DISC recruitment. Cell Death Differ. 15, 773–782 (2008).
Sharp, D.A., Lawrence, D.A. & Ashkenazi, A. Selective knockdown of the long variant of cellular FLICE inhibitory protein augments death receptor-mediated caspase-8 activation and apoptosis. J. Biol. Chem. 280, 19401–19409 (2005).
Krueger, A., Schmitz, I., Baumann, S., Krammer, P.H. & Kirchhoff, S. Cellular FLICE-inhibitory protein splice variants inhibit different steps of caspase-8 activation at the CD95 death-inducing signaling complex. J. Biol. Chem. 276, 20633–20640 (2001).
Boatright, K.M., Deis, C., Denault, J.B., Sutherlin, D.P. & Salvesen, G.S. Activation of caspases-8 and -10 by FLIPL . Biochem. J. 382, 651–657 (2004).
Micheau, O. et al. The long form of FLIP is an activator of caspase-8 at the Fas death-inducing signaling complex. J. Biol. Chem. 277, 45162–45171 (2002).
Varfolomeev, E. et al. c-IAP1 and c-IAP2 are critical mediators of tumor necrosis factor α (TNFα)-induced NF-κB activation. J. Biol. Chem. 283, 24295–24299 (2008).
Bertrand, M.J. et al. cIAP1 and cIAP2 facilitate cancer cell survival by functioning as E3 ligases that promote RIP1 ubiquitination. Mol. Cell 30, 689–700 (2008).
Mahoney, D.J. et al. Both cIAP1 and cIAP2 regulate TNFα-mediated NF-κB activation. Proc. Natl. Acad. Sci. USA 105, 11778–11783 (2008). Together with refs. 33 and 34 , this study implicates c-IAP1/2 as critical E3 ligases involved in RIP1-mediated activation of NF-κB and MAPKs.
Wertz, I.E. et al. De-ubiquitination and ubiquitin ligase domains of A20 downregulate NF-κB signalling. Nature 430, 694–699 (2004).
Liao, W. et al. CARP-2 is an endosome-associated ubiquitin ligase for RIP and regulates TNF-induced NF-κB activation. Curr. Biol. 18, 641–649 (2008).
Hayden, M.S. & Ghosh, S. Shared principles in NF-κB signaling. Cell 132, 344–362 (2008).
Symons, A., Beinke, S. & Ley, S.C. MAP kinase kinase kinases and innate immunity. Trends Immunol. 27, 40–48 (2006).
Varfolomeev, E.E. & Ashkenazi, A. Tumor necrosis factor: an apoptosis JuNKie? Cell 116, 491–497 (2004).
Schutze, S., Tchikov, V. & Schneider-Brachert, W. Regulation of TNFR1 and CD95 signalling by receptor compartmentalization. Nat. Rev. Mol. Cell Biol. 9, 655–662 (2008).
Panka, D.J., Mano, T., Suhara, T., Walsh, K. & Mier, J.W. Phosphatidylinositol 3-kinase/Akt activity regulates c-FLIP expression in tumor cells. J. Biol. Chem. 276, 6893–6896 (2001).
Karin, M., Lawrence, T. & Nizet, V. Innate immunity gone awry: linking microbial infections to chronic inflammation and cancer. Cell 124, 823–835 (2006).
Chang, L. et al. The E3 ubiquitin ligase itch couples JNK activation to TNFα-induced cell death by inducing c-FLIPL turnover. Cell 124, 601–613 (2006).
Stanger, B.Z., Leder, P., Lee, T.H., Kim, E. & Seed, B. RIP: a novel protein containing a death domain that interacts with Fas/APO-1 (CD95) in yeast and causes cell death. Cell 81, 513–523 (1995).
Hsu, H., Huang, J., Shu, H.B., Baichwal, V. & Goeddel, D.V. TNF-dependent recruitment of the protein kinase RIP to the TNF receptor-1 signaling complex. Immunity 4, 387–396 (1996).
O'Donnell, M.A., Legarda-Addison, D., Skountzos, P., Yeh, W.C. & Ting, A.T. Ubiquitination of RIP1 regulates an NF-κB-independent cell-death switch in TNF signaling. Curr. Biol. 17, 418–424 (2007).
Vince, J.E. et al. IAP antagonists target cIAP1 to induce TNFα-dependent apoptosis. Cell 131, 682–693 (2007).
Jin, Z. & El-Deiry, W.S. Distinct signaling pathways in TRAIL- versus tumor necrosis factor-induced apoptosis. Mol. Cell. Biol. 26, 8136–8148 (2006).
Zheng, L. et al. Competitive control of independent programs of tumor necrosis factor receptor-induced cell death by TRADD and RIP1. Mol. Cell. Biol. 26, 3505–3513 (2006).
Park, Y., Lee, S.W. & Sung, Y.C. Cutting edge: CpG DNA inhibits dendritic cell apoptosis by up-regulating cellular inhibitor of apoptosis proteins through the phosphatidylinositide-3′-OH kinase pathway. J. Immunol. 168, 5–8 (2002).
Conte, D. et al. Inhibitor of apoptosis protein cIAP2 is essential for lipopolysaccharide-induced macrophage survival. Mol. Cell. Biol. 26, 699–708 (2006).
Perlman, H. et al. FLICE-inhibitory protein expression during macrophage differentiation confers resistance to fas-mediated apoptosis. J. Exp. Med. 190, 1679–1688 (1999).
Baseta, J.G. & Stutman, O. TNF regulates thymocyte production by apoptosis and proliferation of the triple negative (CD3−CD4−CD8−) subset. J. Immunol. 165, 5621–5630 (2000).
Bidere, N., Su, H.C. & Lenardo, M.J. Genetic disorders of programmed cell death in the immune system. Annu. Rev. Immunol. 24, 321–352 (2006).
Sneller, M.C., Dale, J.K. & Straus, S.E. Autoimmune lymphoproliferative syndrome. Curr. Opin. Rheumatol. 15, 417–421 (2003).
Nagata, S. Apoptosis by death factor. Cell 88, 355–365 (1997).
Green, D.R. Fas Bim boom! Immunity 28, 141–143 (2008).
Goodnow, C.C. Multistep pathogenesis of autoimmune disease. Cell 130, 25–35 (2007).
Chen, M., Huang, L. & Wang, J. Deficiency of Bim in dendritic cells contributes to overactivation of lymphocytes and autoimmunity. Blood 109, 4360–4367 (2007).
Stranges, P.B. et al. Elimination of antigen-presenting cells and autoreactive T cells by Fas contributes to prevention of autoimmunity. Immunity 26, 629–641 (2007). Identifies CD95-dependent elimination of DCs as an important homeostatic mechanism for preventing autoimmunity.
Peter, M.E. et al. The CD95 receptor: apoptosis revisited. Cell 129, 447–450 (2007).
Shortman, K. & Naik, S.H. Steady-state and inflammatory dendritic-cell development. Nat. Rev. Immunol. 7, 19–30 (2007).
Han, L., Zhao, Y. & Jia, X. Mathematical modeling identified c-FLIP as an apoptotic switch in death receptor induced apoptosis. Apoptosis 13, 1198–1204 (2008).
Carey, G.B. et al. B-cell receptor and Fas-mediated signals for life and death. Immunol. Rev. 176, 105–115 (2000).
Kataoka, T. et al. The caspase-8 inhibitor FLIP promotes activation of NF-κB and Erk signaling pathways. Curr. Biol. 10, 640–648 (2000).
Ueffing, N., Schuster, M., Keil, E., Schulze-Osthoff, K. & Schmitz, I. Upregulation of c-FLIPshort by NFAT contributes to apoptosis resistance of short-term activated T cells. Blood 112, 690–698 (2008).
Moriyama, H. & Yonehara, S. Rapid up-regulation of c-FLIP expression by BCR signaling through the PI3K/Akt pathway inhibits simultaneously induced Fas-mediated apoptosis in murine B lymphocytes. Immunol. Lett. 109, 36–46 (2007).
Fluur, C. et al. Potential role for IL-7 in Fas-mediated T cell apoptosis during HIV infection. J. Immunol. 178, 5340–5350 (2007).
Rethi, B. et al. Priming of T cells to Fas-mediated proliferative signals by interleukin-7. Blood 112, 1195–1204 (2008).
Sun, M. & Fink, P.J. A new class of reverse signaling costimulators belongs to the TNF family. J. Immunol. 179, 4307–4312 (2007).
Suzuki, I., Martin, S., Boursalian, T.E., Beers, C. & Fink, P.J. Fas ligand costimulates the in vivo proliferation of CD8+ T cells. J. Immunol. 165, 5537–5543 (2000).
Kokkonen, T.S., Augustin, M.T., Makinen, J.M., Kokkonen, J. & Karttunen, T.J. High endothelial venules of the lymph nodes express Fas ligand. J. Histochem. Cytochem. 52, 693–699 (2004).
Pinkoski, M.J., Brunner, T., Green, D.R. & Lin, T. Fas and Fas ligand in gut and liver. Am. J. Physiol. Gastrointest. Liver Physiol. 278, G354–G366 (2000).
Arase, H., Arase, N. & Saito, T. Fas-mediated cytotoxicity by freshly isolated natural killer cells. J. Exp. Med. 181, 1235–1238 (1995).
Brunner, T. et al. Cell-autonomous Fas (CD95)/Fas-ligand interaction mediates activation-induced apoptosis in T-cell hybridomas. Nature 373, 441–444 (1995).
Norian, L.A. et al. The regulation of CD95 (Fas) ligand expression in primary T cells: induction of promoter activation in CD95LP-Luc transgenic mice. J. Immunol. 164, 4471–4480 (2000).
Kim, S., Iizuka, K., Aguila, H.L., Weissman, I.L. & Yokoyama, W.M. In vivo natural killer cell activities revealed by natural killer cell-deficient mice. Proc. Natl. Acad. Sci. USA 97, 2731–2736 (2000).
Lanier, L.L. Evolutionary struggles between NK cells and viruses. Nat. Rev. Immunol. 8, 259–268 (2008).
Di Santo, J.P. Natural killer cell developmental pathways: a question of balance. Annu. Rev. Immunol. 24, 257–286 (2006).
Huntington, N.D., Vosshenrich, C.A. & Di Santo, J.P. Developmental pathways that generate natural-killer-cell diversity in mice and humans. Nat. Rev. Immunol. 7, 703–714 (2007).
Takeda, K. et al. TRAIL identifies immature natural killer cells in newborn mice and adult mouse liver. Blood 105, 2082–2089 (2005).
Takeda, K. et al. Involvement of tumor necrosis factor-related apoptosis-inducing ligand in surveillance of tumor metastasis by liver natural killer cells. Nat. Med. 7, 94–100 (2001).
Smyth, M.J. et al. Tumor necrosis factor–related apoptosis-inducing ligand (TRAIL) contributes to interferon γ–dependent natural killer cell protection from tumor metastasis. J. Exp. Med. 193, 661–670 (2001).
Seki, N. et al. Tumor necrosis factor-related apoptosis-inducing ligand-mediated apoptosis is an important endogenous mechanism for resistance to liver metastases in murine renal cancer. Cancer Res. 63, 207–213 (2003).
Finnberg, N., Klein-Szanto, A.J. & El-Deiry, W.S. TRAIL-R deficiency in mice promotes susceptibility to chronic inflammation and tumorigenesis. J. Clin. Invest. 118, 111–123 (2008).
Griffith, T.S. et al. Monocyte-mediated tumoricidal activity via the tumor necrosis factor-related cytokine, TRAIL. J. Exp. Med. 189, 1343–1354 (1999).
Washburn, B. et al. TNF-related apoptosis-inducing ligand mediates tumoricidal activity of human monocytes stimulated by Newcastle disease virus. J. Immunol. 170, 1814–1821 (2003).
Fanger, N.A., Maliszewski, C.R., Schooley, K. & Griffith, T.S. Human dendritic cells mediate cellular apoptosis via tumor necrosis factor-related apoptosis-inducing ligand (TRAIL). J. Exp. Med. 190, 1155–1164 (1999).
Sato, K. et al. Antiviral response by natural killer cells through TRAIL gene induction by IFN-α/β. Eur. J. Immunol. 31, 3138–3146 (2001).
Ishikawa, E., Nakazawa, M., Yoshinari, M. & Minami, M. Role of tumor necrosis factor-related apoptosis-inducing ligand in immune response to influenza virus infection in mice. J. Virol. 79, 7658–7663 (2005).
Diehl, G.E. et al. TRAIL-R as a negative regulator of innate immune cell responses. Immunity 21, 877–889 (2004). Implicates mouse DR5 as a negative regulator of innate immune responses by attenuating NF-κB activation.
Hayakawa, Y. et al. NK cell TRAIL eliminates immature dendritic cells in vivo and limits dendritic cell vaccination efficacy. J. Immunol. 172, 123–129 (2004).
Mirandola, P. et al. Activated human NK and CD8+ T cells express both TNF-related apoptosis-inducing ligand (TRAIL) and TRAIL receptors but are resistant to TRAIL-mediated cytotoxicity. Blood 104, 2418–2424 (2004).
Ashkenazi, A. Targeting the extrinsic apoptosis pathway in cancer. Cytokine Growth Factor Rev. 19, 325–331 (2008).
Lavrik, I.N. et al. CD95 stimulation results in the formation of a novel death effector domain protein-containing complex. J. Biol. Chem. 283, 26401–26408 (2008).
Gordon, S. & Taylor, P.R. Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 5, 953–964 (2005).
Johnstone, R.W., Frew, A.J. & Smyth, M.J. The TRAIL apoptotic pathway in cancer onset, progression and therapy. Nat. Rev. Cancer 8, 782–798 (2008).
Lamhamedi-Cherradi, S.-E., Zheng, S.-J., Maguschak, K.A., Peschon, J. & Chen, Y.H. Defective thymocyte apoptosis and accelerated autoimmune diseases in TRAIL−/− mice. Nat. Immunol. 4, 255–260 (2003).
Cretney, E. et al. Normal thymocyte negative selection in TRAIL-deficient mice. J. Exp. Med. 198, 491–496 (2003).
Green, D.R. The suicide in the thymus, a twisted trail. Nat. Immunol. 4, 207–208 (2003).
Janssen, E.M. et al. CD4+ T-cell help controls CD8 T-cell memory via TRAIL-mediated activation-induced cell death. Nature 434, 88–93 (2005). Proposes that 'helpless' CD8+ T cells are eliminated by Apo2L (also called TRAIL), which represents a mechanism for controlling adaptive immune responses.
Hamilton, S.E., Wolkers, M.C., Schoenberger, S.P. & Jameson, S.C. The generation of protective memory-like CD8+ T cells during homeostatic proliferation requires CD4+ T cells. Nat. Immunol. 7, 475–481 (2006).
Weckmann, M. et al. Critical link between TRAIL and CCL20 for the activation of TH2 cells and the expression of allergic airway disease. Nat. Med. 13, 1308–1315 (2007).
Oh, S. et al. IL-15 as a mediator of CD4+ help for CD8+ T cell longevity and avoidance of TRAIL-mediated apoptosis. Proc. Natl. Acad. Sci. USA 105, 5201–5206 (2008).
Sacks, J.A. & Bevan, M.J. TRAIL deficiency does not rescue impaired CD8+ T cell memory generated in the absence of CD4+ T cell help. J. Immunol. 180, 4570–4576 (2008). In contrast to ref. 102 , this study suggests that CD4+ T cell help to CD8+ T cells is not strictly contingent on the prevention of Apo2L (also called TRAIL)-mediated apoptosis.
Ren, X. et al. Involvement of cellular death in TRAIL/DR5-dependent suppression induced by CD4+CD25+ regulatory T cells. Cell Death Differ. 14, 2076–2084 (2007).
Zhang, J., Cado, D., Chen, A., Kabra, N.H. & Winoto, A. Fas-mediated apoptosis and activation-induced T-cell proliferation are defective in mice lacking FADD/Mort1. Nature 392, 296–300 (1998).
Zhang, Y. et al. Conditional Fas-associated death domain protein (FADD): GFP knockout mice reveal FADD is dispensable in thymic development but essential in peripheral T cell homeostasis. J. Immunol. 175, 3033–3044 (2005).
Newton, K., Harris, A.W., Bath, M.L., Smith, K.G. & Strasser, A. A dominant interfering mutant of FADD/MORT1 enhances deletion of autoreactive thymocytes and inhibits proliferation of mature T lymphocytes. EMBO J. 17, 706–718 (1998).
Zornig, M., Hueber, A.O. & Evan, G. p53-dependent impairment of T-cell proliferation in FADD dominant-negative transgenic mice. Curr. Biol. 8, 467–470 (1998).
Salmena, L. et al. Essential role for caspase 8 in T-cell homeostasis and T-cell-mediated immunity. Genes Dev. 17, 883–895 (2003).
Chau, H. et al. Cellular FLICE-inhibitory protein is required for T cell survival and cycling. J. Exp. Med. 202, 405–413 (2005).
Zhang, N., Hopkins, K. & He, Y.W. The long isoform of cellular FLIP is essential for T lymphocyte proliferation through an NF-κB-independent pathway. J. Immunol. 180, 5506–5511 (2008).
Kataoka, T. & Tschopp, J. N-terminal fragment of c-FLIPL processed by caspase 8 specifically interacts with TRAF2 and induces activation of the NF-κB signaling pathway. Mol. Cell. Biol. 24, 2627–2636 (2004).
Dohrman, A. et al. Cellular FLIP (long form) regulates CD8+ T cell activation through caspase-8-dependent NF-κB activation. J. Immunol. 174, 5270–5278 (2005).
Lens, S.M. et al. The caspase 8 inhibitor c-FLIPL modulates T-cell receptor-induced proliferation but not activation-induced cell death of lymphocytes. Mol. Cell. Biol. 22, 5419–5433 (2002).
Zhang, N., Hopkins, K. & He, Y.W. c-FLIP protects mature T lymphocytes from TCR-mediated killing. J. Immunol. 181, 5368–5373 (2008).
Golks, A., Brenner, D., Krammer, P.H. & Lavrik, I.N. The c-FLIP-NH2 terminus (p22-FLIP) induces NF-κB activation. J. Exp. Med. 203, 1295–1305 (2006). Provides a new mechanism by which c-FLIP controls proapoptotic and non-apoptotic signaling in lymphocytes and DCs.
Alappat, E.C., Volkland, J. & Peter, M.E. Cell cycle effects by C-FADD depend on its C-terminal phosphorylation site. J. Biol. Chem. 278, 41585–41588 (2003).
Hua, Z.C., Sohn, S.J., Kang, C., Cado, D. & Winoto, A. A function of Fas-associated death domain protein in cell cycle progression localized to a single amino acid at its C-terminal region. Immunity 18, 513–521 (2003).
Arechiga, A.F. et al. A Fas-associated death domain protein/caspase-8-signaling axis promotes S-phase entry and maintains S6 kinase activity in T cells responding to IL-2. J. Immunol. 179, 5291–5300 (2007).
Misra, R.S. et al. Caspase-8 and c-FLIPL associate in lipid rafts with NF-κB adaptors during T cell activation. J. Biol. Chem. 282, 19365–19374 (2007).
Ch'en, I.L. et al. Antigen-mediated T cell expansion regulated by parallel pathways of death. Proc. Natl. Acad. Sci. USA 105, 17463–17468 (2008). Genetic ablation of caspase-8, NF-κB and RIP1 reveals two forms of cell death that can regulate T-cell proliferation.
Monks, C.R., Kupfer, H., Tamir, I., Barlow, A. & Kupfer, A. Selective modulation of protein kinase C-θ during T-cell activation. Nature 385, 83–86 (1997).
Sun, S.C. & Ley, S.C. New insights into NF-κB regulation and function. Trends Immunol. 29, 469–478 (2008).
Wu, C.J. & Ashwell, J.D. NEMO recognition of ubiquitinated Bcl10 is required for T cell receptor-mediated NF-κB activation. Proc. Natl. Acad. Sci. USA 105, 3023–3028 (2008).
Shambharkar, P.B. et al. Phosphorylation and ubiquitination of the IκB kinase complex by two distinct signaling pathways. EMBO J. 26, 1794–1805 (2007).
Kawadler, H., Gantz, M.A., Riley, J.L. & Yang, X. The paracaspase MALT1 controls caspase-8 activation during lymphocyte proliferation. Mol. Cell 31, 415–421 (2008). The paracaspase domain of MALT1 (of the CBM complex) induces caspase-8 activation through a direct interaction. Implication of c-FLIP/caspase-8 association in TCR-mediated NF-κB activation.
Liu, Y.C. The E3 ubiquitin ligase Itch in T cell activation, differentiation, and tolerance. Semin. Immunol. 19, 197–205 (2007).
Meylan, E. et al. RIP1 is an essential mediator of Toll-like receptor 3-induced NF-κB activation. Nat. Immunol. 5, 503–507 (2004).
Cusson-Hermance, N., Khurana, S., Lee, T.H., Fitzgerald, K.A. & Kelliher, M.A. Rip1 mediates the Trif-dependent toll-like receptor 3- and 4-induced NF-κB activation but does not contribute to interferon regulatory factor 3 activation. J. Biol. Chem. 280, 36560–36566 (2005).
Yamamoto, M. et al. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science 301, 640–643 (2003).
Sato, S. et al. Toll/IL-1 receptor domain-containing adaptor inducing IFN-β (TRIF) associates with TNF receptor-associated factor 6 and TANK-binding kinase 1, and activates two distinct transcription factors, NF-κB and IFN-regulatory factor-3, in the Toll-like receptor signaling. J. Immunol. 171, 4304–4310 (2003).
Yoshida, R. et al. TNF receptor-associated factor (TRAF) 6 and MEK kinase (MEKK) 1 play a pivotal role in the retinoic-acid-inducible gene-I (RIG-I)-like helicase antiviral pathway. J. Biol. Chem. 283, 36211–36220 (2008).
Maelfait, J. et al. Stimulation of Toll-like receptor 3 and 4 induces interleukin-1β maturation by caspase-8. J. Exp. Med. 205, 1967–1973 (2008). Suggests a new role for caspase-8 in the production of biologically active IL-1β in response to TLR3 and TLR4 stimulation.
Kaiser, W.J. & Offermann, M.K. Apoptosis induced by the toll-like receptor adaptor TRIF is dependent on its receptor interacting protein homotypic interaction motif. J. Immunol. 174, 4942–4952 (2005).
Sun, H. et al. TIPE2, a negative regulator of innate and adaptive immunity that maintains immune homeostasis. Cell 133, 415–426 (2008). Identifies TIPE2 as an important negative regulator of caspase-8–mediated proinflammatory activity.
Pietras, E.M. & Cheng, G. A new TRADDition in intracellular antiviral signaling. Sci. Signal. 1, pe36 (2008).
Oganesyan, G. et al. Critical role of TRAF3 in the Toll-like receptor-dependent and -independent antiviral response. Nature 439, 208–211 (2006).
Saha, S.K. et al. Regulation of antiviral responses by a direct and specific interaction between TRAF3 and Cardif. EMBO J. 25, 3257–3263 (2006).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
V.D., N.S.W. and A.A. are employees of Genentech, Inc.
Rights and permissions
About this article
Cite this article
Wilson, N., Dixit, V. & Ashkenazi, A. Death receptor signal transducers: nodes of coordination in immune signaling networks. Nat Immunol 10, 348–355 (2009). https://doi.org/10.1038/ni.1714
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.1714
This article is cited by
-
PANoptosis: a potential new target for programmed cell death in breast cancer treatment and prognosis
Apoptosis (2024)
-
THADA inhibition in mice protects against type 2 diabetes mellitus by improving pancreatic β-cell function and preserving β-cell mass
Nature Communications (2023)
-
Caspase-8 auto-cleavage regulates programmed cell death and collaborates with RIPK3/MLKL to prevent lymphopenia
Cell Death & Differentiation (2022)
-
Mosaic composition of RIP1–RIP3 signalling hub and its role in regulating cell death
Nature Cell Biology (2022)
-
Phosphoproteome profiling uncovers a key role for CDKs in TNF signaling
Nature Communications (2021)