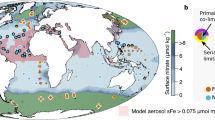
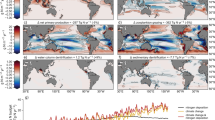
Abstract
Microbial activity is a fundamental component of oceanic nutrient cycles. Photosynthetic microbes, collectively termed phytoplankton, are responsible for the vast majority of primary production in marine waters. The availability of nutrients in the upper ocean frequently limits the activity and abundance of these organisms. Experimental data have revealed two broad regimes of phytoplankton nutrient limitation in the modern upper ocean. Nitrogen availability tends to limit productivity throughout much of the surface low-latitude ocean, where the supply of nutrients from the subsurface is relatively slow. In contrast, iron often limits productivity where subsurface nutrient supply is enhanced, including within the main oceanic upwelling regions of the Southern Ocean and the eastern equatorial Pacific. Phosphorus, vitamins and micronutrients other than iron may also (co-)limit marine phytoplankton. The spatial patterns and importance of co-limitation, however, remain unclear. Variability in the stoichiometries of nutrient supply and biological demand are key determinants of oceanic nutrient limitation. Deciphering the mechanisms that underpin this variability, and the consequences for marine microbes, will be a challenge. But such knowledge will be crucial for accurately predicting the consequences of ongoing anthropogenic perturbations to oceanic nutrient biogeochemistry.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Frausto da Silva, J. J. R. & Williams, R. J. P. The Biological Chemistry of the Elements: The Inorganic Chemistry of Life, 2nd edn (Oxford Univ. Press, 2001).
Sterner, R. W. & Elser, J. J. Ecological Stoichiometry: The Biology of Elements from Molecules to the Biosphere (Princeton Univ. Press, 2002).
Redfield, A. C. in James Johnstone Memorial Volume, 176–192 (Liverpool Univ. Press, 1934).
Redfield, A. C., The biological control of chemical factors in the environment. Am. Sci. 46, 205–221 (1958).
de Baar, H. J. W., von Liebig's law of the minimum and plankton ecology (1899–1991). Prog. Oceanogr. 33, 347–386 (1994).
Morel, F. M. M., Milligan, A. J. & Saito, M. A. Marine Bioinorganic Chemistry: The Role of Trace Metals in the Ocean Cycles of Major Nutrients. Treatise on Geochemistry Vol. 6, 113–143 (2003).
Arrigo, K. R. Marine microorganisms and global nutrient cycles. Nature 437, 349–355 (2005).
Saito, M. A., Goepfert, T. J. & Ritt, J. T. Some thoughts on the concept of colimitation: Three definitions and the importance of bioavailability. Limnol. Oceanogr. 53, 276–290 (2008).
Geider, R. J. & La Roche, J. Redfield revisited: variability of C:N:P in marine microalgae and its biochemical basis. Eur. J. Phycol. 37, 1–17 (2002).
Quigg, A. et al. The evolutionary inheritance of elemental stoichiometry in marine phytoplankton. Nature 425, 291–294 (2003).
Klausmeier, C. A., Litchman, E., Daufresne, T. & Levin, S. A. Phytoplankton stoichiometry. Ecol. Res. 23, 479–485 (2008).
Duce, R. A. et al. Impacts of atmospheric anthropogenic nitrogen on the open ocean. Science 320, 893–897 (2008).
Krishnamurthy, A. et al. Impacts of increasing anthropogenic soluble iron and nitrogen deposition on ocean biogeochemistry. Glob. Biogeochem. Cycles 23, GB3016 (2009).
Falkowski, P. et al. The global carbon cycle: A test of our knowledge of Earth as a system. Science 290, 291–296 (2000).
Raven, J. A. Contributions of anoxygenic and oxygenic phototrophy and chemolithotrophy to carbon and oxygen fluxes in aquatic environments. Aquat. Microb. Ecol. 56, 177–192 (2009).
Eppley, R. W. & Peterson, B. J. Particulate organic matter flux and planktonic new production in the deep ocean. Nature 282, 677–680 (1979).
Blackman, F. F. Optima and limiting factors. Ann. Bot. 19, 281–298 (1905).
Cullen, J. J. Hypotheses to explain high-nutrient conditions in the open sea. Limnol. Oceanogr. 36, 1578–1599 (1991).
von Liebig, J., Chemistry and its Application to Agriculture And Physiology. (Taylor and Walton, London, 1840).
Thingstad, T. F. et al. Nature of phosphorus limitation in the ultraoligotrophic eastern Mediterranean. Science 309, 1068–1071 (2005).
Boyd, P. W., Strzepek, R., Fu, F. X. & Hutchins, D. A. Environmental control of open-ocean phytoplankton groups: Now and in the future. Limnol. Oceanogr. 55, 1353–1376 (2010).
Cavender-Bares, K. K., Mann, E. L., Chisholm, S. W., Ondrusek, M. E. & Bidigare, R. R. Differential response of equatorial Pacific phytoplankton to iron fertilisation. Limnol. Oceanogr. 44, (1999).
Chisholm, S. W. in Primary Productivity and Biogeochemical Cycles in the Sea (eds Falkowski, P. G. & Woodhead, A. D.) 213–237 (Plenum, 1992).
Raven, J. A. The twelfth Tansley lecture. Small is beautiful: The picophytoplankton. Funct. Ecol. 12, 503–513 (1998).
Van Mooy, B. A. S. et al. Phytoplankton in the ocean use non-phosphorus lipids in response to phosphorus scarcity. Nature 458, 69–72 (2009).
Irigoien, X., Huisman, J. & Harris, R. P. Global biodiversity patterns of marine phytoplankton and zooplankton. Nature 429, 863–867 (2004).
Tyrrell, T. The relative influences of nitrogen and phosphorus on oceanic primary production. Nature 400, 525–531 (1999).
Falkowski, P. G. Evolution of the nitrogen cycle and its influence on the biological sequestration of CO2 in the ocean. Nature 387, 272–275 (1997).
Mills, M. M., Ridame, C., Davey, M., La Roche, J. & Geider, R. J. Iron and phosphorus co-limit nitrogen fixation in the eastern tropical North Atlantic. Nature 429, 292–294 (2004).
Moore, J. K. & Doney, S. C. Iron availability limits the ocean nitrogen inventory stabilizing feedbacks between marine denitrification and nitrogen fixation. Glob. Biogeochem. Cycles 21, GB2001 (2007).
Moore, C. M. et al. Large-scale distribution of Atlantic nitrogen fixation controlled by iron availability. Nature Geosci. 2, 867–871 (2009).
Dupont, C. L., Butcher, A., Valas, R. E., Bourne, P. E. & Caetano-Anolles, G. History of biological metal utilization inferred through phylogenomic analysis of protein structures. Proc. Natl Acad. Sci. USA 107, 10567–10572 (2010).
Anderson, L. A. & Sarmiento, J. L. Redfield ratios of remineralization determined by nutrient data-analysis. Glob. Biogeochem. Cycles 8, 65–80 (1994).
Deutsch, C., Sarmiento, J. L., Sigman, D. M., Gruber, N. & Dunne, J. P. Spatial coupling of nitrogen inputs and losses in the ocean. Nature 445, 163–167 (2007).
Ho, T. Y. et al. The elemental composition of some marine phytoplankton. J. Phycol. 39, 1145–1159 (2003).
Sunda, W. G. & Huntsman, S. A. Cobalt and zinc interreplacement in marine phytoplankton: Biological and geochemical implications. Limnol. Oceanogr. 40, 1404–1417 (1995).
Sunda, W. G. & Huntsman, S. A. Interrelated influence of iron, light and cell size on marine phytoplankton growth. Nature 390, 389–392 (1997).
Sunda, W. G. & Huntsman, S. A. Control of Cd concentrations in a coastal diatom by interactions among free ionic Cd, Zn, and Mn in seawater. Environ. Sci. Technol. 32, 2961–2968 (1998).
Saito, M. A., Sigman, D. M. & Morel, F. M. M. The bioinorganic chemistry of the ancient ocean: The co-evolution of cyanobacterial metal requirements and biogeochemical cycles at the Archean–Proterozoic boundary? Inorg. Chim. Acta 356, 308–318 (2003).
Grzymski, J. J. & Dussaq, A. M. The significance of nitrogen cost minimization in proteomes of marine microorganisms. ISME J. 6, 71–80 (2012).
Wu, J., Sunda, W., Boyle, E. A. & Karl, D. M. Phosphate depletion in the Western North Atlantic Ocean. Science 289, 759–762 (2000).
Lenton, T. M. & Klausmeier, C. A. Biotic stoichiometric controls on the deep ocean N:P ratio. Biogeosciences 4, 353–367 (2007).
Sarmiento, J. L., Gruber, N., Brezinski, M. A. & Dunne, J. P. High-latitude controls of thermocline nutrients and low latitude biological productivity. Nature 427, 56–60 (2004).
Deutsch, C. & Weber, T. Nutrient ratios as a tracer and driver of ocean biogeochemistry. Annu. Rev. Mar. Sci. 4, 113–141 (2012).
Moore, J. K., Doney, S. C., Glover, D. M. & Fung, I. Y. Iron cycling and nutrient-limitation patterns in surface waters of the World Ocean. Deep-Sea Res. II Top. Studies Oceanogr. 49, 463–507 (2002).
Krishnamurthy, A., Moore, J. K., Mahowald, N., Luo, C. & Zender, C. S. Impacts of atmospheric nutrient inputs on marine biogeochemistry. J. Geophys. Res. 115, G01006 (2010).
Moore, C. M. et al. Relative influence of nitrogen and phosphorus availability on phytoplankton physiology and productivity in the oligotrophic sub-tropical North Atlantic Ocean. Limnol. Oceanogr. 53, 291–305 (2008).
Tanaka, T. et al. Lack of P-limitation of phytoplankton and heterotrophic prokaryotes in surface waters of three anticyclonic eddies in the stratified Mediterranean Sea. Biogeosciences 8, 525–538 (2011).
Gruber, N. & Galloway, J. N. An Earth-system perspective of the global nitrogen cycle. Nature 451, 293–296 (2008).
Flynn, K. J. Ecological modelling in a sea of variable stoichiometry: Dysfunctionality and the legacy of Redfield and Monod. Prog. Oceanogr. 84, 52–65 (2010).
Steinacher, M. et al. Projected 21st century decrease in marine productivity: A multi-model analysis. Biogeosciences 7, 979–1005 (2010).
Moutin, T. et al. Phosphate availability and the ultimate control of new nitrogen input by nitrogen fixation in the tropical Pacific Ocean. Biogeosciences 5, 95–109 (2008).
Martin, J. H. & Fitzwater, S. E. Iron deficiency limits phytoplankton growth in the north-east Pacific subarctic. Nature 331, 341–343 (1988).
Boyd, P. W. et al. Mesoscale iron enrichment experiments 1993–2005: Synthesis and future directions. Science 315, 612–617 (2007).
Menzel, D. W. & Ryther, J. H. Nutrients limiting the production of phytoplankton in the Sargasso Sea, with special reference to iron. Deep-Sea Res. 7, 276–281 (1961).
Graziano, L. M., Geider, R. J., Li, W. K. W. & Olaizola, M. Nitrogen limitation of North Atlantic phytoplankton: Analysis of physiological condition in nutrient enrichment experiments. Aquat. Microb. Ecol. 11, 53–64 (1996).
Dyhrman, S. T., Webb, E. A., Anderson, D. M., Moffett, J. W. & Waterbury, J. B. Cell-specific detection of phosphorus stress in Trichodesmium from the western north Atlantic. Limnol. Oceanogr. 47, 1832–1836 (2002).
Lomas, M. W., Swain, A., Shelton, R. & Ammerman, J. W. Taxonomic variability of phosphorus stress in Sargasso Sea phytoplankton. Limnol. Oceanogr. 49, 2303–2310 (2004).
Zohary, T. et al. P-limited bacteria but N and P co-limited phytoplankton in the Eastern Mediterranean: A microcosm experiment. Deep-Sea Res. II 52, 3011–3023 (2005).
La Roche, J., Geider, R. J., Graziano, L. M., Murray, H. & Lewis, K. Induction of specific proteins in eukaryotic algae grown under iron-, phosphorus-, or nitrogen-deficient conditions. J. Phycol. 29, 767–777 (1993).
Chappell, P. D., Moffett, J. W., Hynes, A. M. & Webb, E. A. Molecular evidence of iron limitation and availability in the global diazotroph Trichodesmium. ISME J. 6, 1728–1739 (2012).
Marchetti, A. et al. Comparative metatranscriptomics identifies molecular bases for the physiological responses of phytoplankton to varying iron availability. Proc. Natl Acad. Sci. USA 109, E317–E325 (2012).
Sigman, D. M., Hain, M. P. & Haug, G. H. The polar ocean and glacial cycles in atmospheric CO2 concentration. Nature 466, 47–55 (2010).
Martinez-Garcia, A. et al. Southern Ocean dust–climate coupling over the past four million years. Nature 476, 312–315 (2011).
Ren, H. et al. Foraminiferal isotope evidence of reduced nitrogen fixation in the ice age Atlantic Ocean. Science 323, 244–248 (2009).
Noble, A. E. et al. Basin-scale inputs of cobalt, iron, and manganese from the Benguela–Angola front to the South Atlantic Ocean. Limnol. Oceanogr. 57, 989–1010 (2012).
Shi, D. L., Xu, Y., Hopkinson, B. M. & Morel, F. M. M. Effect of ocean acidification on iron availability to marine phytoplankton. Science 327, 676–679 (2010).
Beman, J. M. et al. Global declines in oceanic nitrification rates as a consequence of ocean acidification. Proc. Natl Acad. Sci. USA 108, 208–213 (2011).
Sunda, W. G. Iron and the carbon pump. Science 327, 654–655 (2010).
Sarmiento, J. L., Hughes, T. M. C., Stouffer, R. J. & Manabe, S. Simulated response of the ocean carbon cycle to anthropogenic climate warming. Nature 393, 245–249 (1998).
Sarmiento, J. L. et al. Response of ocean ecosystems to climate warming. Glob. Biogeochem. Cycles 18, GB3003 (2004).
Polovina, J. J., Howell, E. A. & Abecassis, M. Ocean's least productive waters are expanding. Geophys. Res. Lett. 35, L03618 (2008).
Saba, V. S. et al. Challenges of modeling depth-integrated marine primary productivity over multiple decades: A case study at BATS and HOT. Glob. Biogeochem. Cycles 24, GB3020 (2010).
Henson, S. A. et al. Detection of anthropogenic climate change in satellite records of ocean chlorophyll and productivity. Biogeosciences 7, 621–640 (2010).
Stramma, L., Johnson, G. C., Sprintall, J. & Mohrholz, V. Expanding oxygen-minimum zones in the tropical oceans. Science 320, 655–658 (2008).
Godfray, H. C. J. et al. Food security: The challenge of feeding 9 billion people. Science 327, 812–818 (2010).
Mahowald, N. et al. Global distribution of atmospheric phosphorus sources, concentrations and deposition rates, and anthropogenic impacts. Glob. Biogeochem. Cycles 22, GB4026 (2008).
Mahowald, N. M. et al. Observed 20th century desert dust variability: Impact on climate and biogeochemistry. Atmos. Chem. Phys. 10, 10875–10893 (2010).
Seitzinger, S. P. et al. Global river nutrient export: A scenario analysis of past and future trends. Glob. Biogeochem. Cycles 24, GB0A08 (2010).
Cordell, D., Drangert, J. O. & White, S. The story of phosphorus: Global food security and food for thought. Glob. Environ. Change Hum. Policy Dimens. 19, 292–305 (2009).
Raiswell, R. & Canfield, D. E. The iron biogeochemical cycle past and present geochemical perspectives. Geochem. Persp. 1, 1–220 (2012).
Jickells, T. D. et al. Global iron connections between desert dust, ocean biogeochemistry, and climate. Science 308, 67–71 (2005).
Monteiro, F. M., Dutkiewicz, S. & Follows, M. J. Biogeographical controls on the marine nitrogen fixers. Glob. Biogeochem. Cycles 25, GB2003 (2011).
Diaz, R. J. & Rosenberg, R. Spreading dead zones and consequences for marine ecosystems. Science 321, 926–929 (2008).
Levitan, O. et al. Elevated CO2 enhances nitrogen fixation and growth in the marine cyanobacterium Trichodesmium. Glob. Change Biol. 13, 531–538 (2007).
Eppley, R. W. Temperature and phytoplankton growth in the sea. Fishery Bull. 70, 1063–1085 (1972).
Shi, D., Kranz, S. A., Kim, J-M. & Morel, F. M. M. Ocean acidification slows nitrogen fixation and growth in the dominant diazotroph Trichodesmium under low-iron conditions. Proc. Natl Acad. Sci. USA 109, E3094–3100 (2012).
Taucher, J. & Oschlies, A. Can we predict the direction of marine primary production change under global warming? Geophys. Res. Lett. 38, 6 (2011).
Breitbarth, E., Oschlies, A. & LaRoche, J. Physiological constraints on the global distribution of Trichodesmium—effect of temperature on diazotrophy. Biogeosciences 4, 53–61 (2007).
Marinov, I. et al. Impact of oceanic circulation on biological carbon storage in the ocean and atmospheric pCO2 . Glob. Biogeochem. Cycles 22, GB3007 (2008).
Marinov, I., Gnanadesikan, A., Toggweiler, J. R. & Sarmiento, J. L. The Southern Ocean biogeochemical divide. Nature 441, 964–967 (2006).
Ito, T. & Follows, M. J. Preformed phosphate, soft tissue pump and atmospheric CO2 . J. Mar. Res. 63, 813–839 (2005).
Sarmiento, J. L. & Toggweiler, J. R. A new model for the role of the oceans in determining atmospheric pCO2 . Nature 308, 621–624 (1984).
Mills, M. M. & Arrigo, K. R. Magnitude of oceanic nitrogen fixation influenced by the nutrient uptake ratio of phytoplankton. Nature Geosci. 3, 412–416 (2010).
Henderson, G. M. et al. GEOTRACES—An international study of the global marine biogeochemical cycles of trace elements and their isotopes. Chem. Erde Geochem. 67, 85–131 (2007).
Raiswell, R. et al. Contributions from glacially derived sediment to the global iron (oxyhydr)oxide cycle: Implications for iron delivery to the oceans. Geochim. Cosmochim. Acta 70, 2765–2780 (2006).
Wynn, P. M., Hodson, A. J., Heaton, T. H. E. & Chenery, S. R. Nitrate production beneath a high arctic glacier, Svalbard. Chem. Geol. 244, 88–102 (2007).
Wallmann, K. Phosphorus imbalance in the global ocean? Glob. Biogeochem. Cycles 24, GB4030 (2010).
Cullen, J. J., Yang, X. & MacIntyre, H. L. in Primary Productivity and Biogeochemical Cycles in the Sea (eds Falkowski, P. G. & Woodhead, A.) 69–88 (Plenum, 1992).
Thingstad, T. F., Ovreas, L., Egge, J. K., Lovdal, T. & Heldal, M. Use of non-limiting substrates to increase size; a generic strategy to simultaneously optimize uptake and minimize predation in pelagic osmotrophs? Ecol. Lett. 8, 675–682 (2005).
Acknowledgements
This review results from the activities of the International Geosphere–Biosphere Programme (IGBP) Fast Track Initiative on Upper Ocean Nutrient Limitation and in particular a workshop hosted at the National Oceanography Centre, Southampton, UK. Financial support for the workshop was provided by IGBP, US Ocean Carbon and Biogeochemistry, the Scientific Committee on Oceanic Research (SCOR) and EU-COST-735.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Regulation of marine phytoplankton by two nutrient limitation regimes (PDF 5654 kb)
Rights and permissions
About this article
Cite this article
Moore, C., Mills, M., Arrigo, K. et al. Processes and patterns of oceanic nutrient limitation. Nature Geosci 6, 701–710 (2013). https://doi.org/10.1038/ngeo1765
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ngeo1765
This article is cited by
-
Diatom-mediated food web functioning under ocean artificial upwelling
Scientific Reports (2024)
-
The impacts of climate change on coastal groundwater
Nature Reviews Earth & Environment (2024)
-
Palaeozoic cooling modulated by ophiolite weathering through organic carbon preservation
Nature Geoscience (2024)
-
Spatial and temporal variation in surface nitrate and phosphate in the Northern Gulf of Mexico over 35 years
Scientific Reports (2024)
-
Atmospheric deposition and river runoff stimulate the utilization of dissolved organic phosphorus in coastal seas
Nature Communications (2024)