Abstract
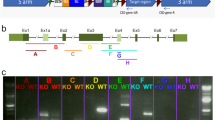
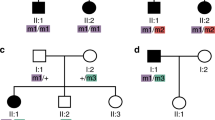
Oguchi disease is a rare autosomal recessive form of congenital stationary night blindness with all other visual functions, including visual acuity, visual field, and colour vision being usually normal1–3. A typical clinical feature of the disorder is a golden or gray-white discolouration of the fundus which disappears in the dark-adapted state and reappears shortly after the onset of light (‘Mizuo phenomenon’; Fig. 1)4. The course of dark adaptation of rod photoreceptors is extremely retarded in Oguchi disease while that of cones appears to proceed normally. The locus for Oguchi disease was recently mapped between D2S172 and D2S345 on distal chromosome 2q by linkage analysis5. Interestingly, the gene for arrestin, an intrinsic rod photoreceptor protein implicated in the recovery phase of light transduction, also maps to this region of chromosome 2q (refs 6,7). Here we report that in five out of six unrelated Japanese patients with Oguchi disease, we have identified a homozygous deletion of nucleotide 1147 (1147delA) in codon 309 of the arrestin gene, predicting a shift in the reading frame and a premature termination of translation which may result in ‘functional null alleles’.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Oguchi, C. Über eine Abart von Hemeralopie. Acta Soc. Ophthalmol. Jpn. 11, 123–134 (1907).
Carr, R.E. & Gouras, P. Oguchi disease. Arch. Ophthalmol. 73, 646–656 (1965).
Carr, R.E. & Ripps, H. Rhodopsin kinetics and rod adaptation in Oguchi disease. Invest. Ophthalmol. 6, 426–436 (1991).
Mizuo, G. A new discovery in dark adaptation in Oguchi disease. Acta Soc. Ophthalmol. Jpn. 17, 1148–1150 (1913).
Maw, M.A. et al. Oguchi disease: suggestion of linkage to markers on chromosome 2q. J. med. Genet. 32, 396–398 (1995).
Lu-Kuo, J., Ward, D.C. & Spritz, R.A. Fluorescence in situ hybridization mapping of 25 markers on distal human chromosome 2q surrounding the human Waardenburg syndrome type I (WS1) locus (PAX3 gene). Genomics 16, 173–179 (1993).
Valverde, D. et al. Genetic fine localization of the arrestin (S-antigen) gene 4 cM distal from D2S172. Hum. Genet. 94, 193–194 (1994).
Kühn, H., Hall, S.W. & Wilden, U. Light induced binding of 48-kDa protein to photoreceptor membranes is highly enhanced by phosphorylation of rhodopsin. FEBS Lett. 176, 473–478 (1984).
Wilden, U., Hall, S.W. & Kühn, H. Phoshodiesterase activation by photoexcited rhodopsin is quenched when rhodopsin is phosphorylated and binds the intrinsic 48-kDa protein of rod outer segments. Proc. natn. Acad. Sci. U.S.A. 83, 1174–1178 (1986).
Palczewski, K., Rispoli, G. & Detwiler, P.B. The influence of arrestin (48K protein) and rhodopsin kinase on visual transduction. Neuron 8, 117–126 (1992).
Palczewski, K., McDowell, J.H., Jakes, S., Ingebritsen, T.S. & Hargrave, P.A. Regulation of rhodopsin dephosphorylation by arrestin. J. biol. Chem. 264, 15770–15773 (1989).
Yamaki, K., Tsuda, M. & Shinohara, T. The sequence of human retinal S-antigen reveals similarities with α-transducin. FEBS Lett. 234, 39–43 (1988).
Yamaki, K., Tsuda, M., Kikuchi, T., Chen, K.-H., Huang, K.-P. & Shinohara, T. Structural organization of the human S-antigen gene. J. biol. Chem. 265, 20757–20762 (1990).
Sheffield, V.C., Beck, J.S., Nichols, B., Lidral, C.E.M. Detection of multiallelic polymorphisms within gene sequences by GC-clamped denaturing gradient gel electrophoresis. Am. J. hum. Genet. 50, 567–575 (1992).
Gal, A., Orth, U., Baehr, W., Schwinger, E. & Rosenberg, T. Heterozygous missense mutation in the rod cGMP phosphodiesterase β-subunit gene in autosomal dominant stationary night blindness. Nature Genet. 7, 64–68 (1994).
Dryja, T.P., Berson, E.L., Rao, V.R. & Oprian, D.D. Heterozygous missense mutation in the rhodopsin gene as a cause of congenital stationary night blindness. Nature Genet. 4, 280–283 (1993).
Rosenfeld, P.J. et al. A null mutation in the rhodopsin gene causes rod photoreceptor dysfunction and autosomal recessive retinitis pigmentosa. Nature Genet. 1, 209–213 (1992).
McLaughlin, M.E., Sand berg, M A., Berson, E.L & Dryja, T.D. Recessive mutations in the gene encoding the β-subunit of rod phosphodiesterase in patients with retinitis pigmentosa. Nature Genet. 4, 130–133 (1993).
Dolph, P.J., Ranganathan, R., Colley, N.J., Hardy, R.W., Socolich, M. & Zuker, C.S. Arrestin function in inactivation of G protein-coupled receptor rhodopsin in vivo. Science. 260, 1910–1916 (1993).
de Jong, R.T.V.M., Zrenner, E., van Meel, G.J., Kennen, J.E.E. & van Norren, D. Mizuo phenomenon in X-linked retinoschisis. Arch. Ophthalmol. 109, 1104–1108 (1991).
Heckenlively, J.R. & Weleber, R.G. X-linked recessive cone dystrophy with tapetal-like sheen: a newly recognize entity with Mizuo-Nakamura phenomenon. Arch. Ophthalmol. 104, 1322–1328 (1986).
Orita, M., Suzuki, Y., Sekyia, T. & Hayashi, K. Rapid and sensitive detection of point mutations and DNA polymorphisms using the polymerase chain reaction. Genomics 5, 874–879 (1989).
Budowle, B., Chakraborty, R., Ginsti, A., Eisenberg, A. & Alien, R. Analysis of the VNTR Locus D1S80 by the PCR followed by high-resolution PAGE. Am. J. hum. Genet. 48, 137–144 (1991).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Fuchs, S., Nakazawa, M., Maw, M. et al. A homozygous 1–base pair deletion in the arrestin gene is a frequent cause of Oguchi disease in Japanese. Nat Genet 10, 360–362 (1995). https://doi.org/10.1038/ng0795-360
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ng0795-360
This article is cited by
-
Central serous chorioretinopathy with a golden sheen
Eye (2024)
-
Genetic analysis and clinical features of three Chinese patients with Oguchi disease
Documenta Ophthalmologica (2023)
-
Mutation analysis reveals novel and known mutations in SAG gene in first two Egyptian families with Oguchi disease
BMC Ophthalmology (2022)
-
A compound heterozygous mutation in the S-Antigen Visual Arrestin SAG gene in a Chinese patient with Oguchi type one: a case report
BMC Ophthalmology (2022)
-
Negative electroretinograms: genetic and acquired causes, diagnostic approaches and physiological insights
Eye (2021)