Abstract
The shortage of donors for organ transplantation has stimulated research on stem cells as a potential resource for cell-based therapy in all human tissues. Stem cells have been used for regenerative medicine applications in many organ systems, including the genitourinary system. The potential applications for stem cell therapy have, however, been restricted by the ethical issues associated with embryonic stem cell research. Instead, scientists have explored other cell sources, including progenitor and stem cells derived from adult tissues and stem cells derived from the amniotic fluid and placenta. In addition, novel techniques for generating stem cells in the laboratory are being developed. These techniques include somatic cell nuclear transfer, in which the nucleus of an adult somatic cell is placed into an oocyte, and reprogramming of adult cells to induce stem-cell-like behavior. Such techniques are now being used in tissue engineering applications, and some of the most successful experiments have been in the field of urology. Techniques to regenerate bladder tissue have reached the clinic, and exciting progress is being made in other areas, such as regeneration of the kidney and urethra. Cell therapy as a treatment for incontinence and infertility might soon become a reality. Physicians should be optimistic that regenerative medicine and tissue engineering will one day provide mainstream treatment options for urologic disorders.
Key Points
-
Regenerative medicine and tissue engineering strategies are becoming a viable option for replacing diseased or damaged organs, particularly in the urinary tract
-
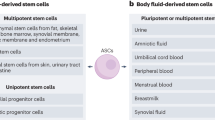
Adult stem cells would be an ideal source of autologous cells for production of new organs, but because they can be difficult to isolate and expand in vitro, their use has been limited
-
Embryonic stem cells provide an almost limitless source of cells for regenerative applications, but ethical concerns limit their use; thus, techniques such as somatic cell nuclear transfer and reprogramming are being developed so that embryonic-like stem cells can be obtained without the destruction of an embryo
-
Amniotic fluid and placenta have been shown to contain pluripotent cells that can be driven to differentiate into a wide variety of specific cell types, and these cells might be useful for tissue engineering applications
-
Tissue engineering strategies for regenerating components of the urinary tract, such as the kidney, bladder, and urethra, are extremely promising; tissue engineering strategies for repairing urethral tissue are in clinical use, and laboratory-grown bladders have been implanted into patients
-
While more study is needed, regenerative medicine and tissue engineering might one day produce mainstream treatments for organ failure
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Weiner LP (2008) Definitions and criteria for stem cells. Methods Mol Biol 438: 3–8
Jain KK (2005) Ethical and regulatory aspects of embryonic stem cell research. Expert Opin Biol Ther 5: 153–162
Ballas CB et al. (2002) Adult bone marrow stem cells for cell and gene therapies: implications for greater use. J Cell Biochem Suppl 38: 20–28
Jiao J and Chen DF (2008) Induction of neurogenesis in nonconventional neurogenic regions of the adult central nervous system by niche astrocyte-produced signals. Stem Cells 26: 1221–1230
Taupin P (2006) Therapeutic potential of adult neural stem cells. Recent Patents CNS Drug Discov 1: 299–303
Jensen UB et al. (2008) A distinct population of clonogenic and multipotent murine follicular keratinocytes residing in the upper isthmus. J Cell Sci 121: 609–617
Crisan M et al. (2008) Purification and long-term culture of multipotent progenitor cells affiliated with the walls of human blood vessels: myoendothelial cells and pericytes. Methods Cell Biol 86: 295–309
Devine SM (2002) Mesenchymal stem cells: will they have a role in the clinic? J Cell Biochem Suppl 38: 73–79
Jiang Y et al. (2002) Pluripotency of mesenchymal stem cells derived from adult marrow. Nature 418: 41–49
Spradling A et al. (2001) Stem cells find their niche. Nature 414: 98–104
Caplan AI (2007) Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol 213: 341–347
da Silva Meirelles L et al. (2008) In search of the in vivo identity of mesenchymal stem cells. Stem Cells [10.1634/stemcells.2007-1122]
Duan X et al. (2007) Disrupted-In-Schizophrenia 1 regulates integration of newly generated neurons in the adult brain. Cell 130: 1146–1158
Crisan M et al. (2008) A reservoir of brown adipocyte progenitors in human skeletal muscle. Stem Cells [10.1634/stemcells.2008-0325]
Luttun A et al. (2006) Differentiation of multipotent adult progenitor cells into functional endothelial and smooth muscle cells. Curr Protoc Immunol 22: Unit 22F.9
Mimeault M and Batra SK (2008) Recent progress on tissue-resident adult stem cell biology and their therapeutic implications. Stem Cell Rev 4: 27–49
Ikeda E et al. (2008) Multipotent cells from the human third molar: feasibility of cell-based therapy for liver disease. Differentiation 76: 495–505
Nolen-Walston RD et al. (2008) Cellular kinetics and modeling of bronchioalveolar stem cell response during lung regeneration. Am J Physiol Lung Cell Mol Physiol 294: L1158–L1165
In't Anker PS et al. (2003) Mesenchymal stem cells in human second-trimester bone marrow, liver, lung, and spleen exhibit a similar immunophenotype but a heterogeneous multilineage differentiation potential. Haematologica 88: 845–852
Hristov M et al. (2008) Adult progenitor cells in vascular remodeling during atherosclerosis. Biol Chem 389: 837–844
Martin GR (1981) Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci USA 78: 7634–7638
Thomson JA et al. (1998) Embryonic stem cell lines derived from human blastocysts. Science 282: 1145–1147
Polgár K et al. (1989) Characterization of rapidly adhering amniotic fluid cells by combined immunofluorescence and phagocytosis assays. Am J Hum Genet 45: 786–792
Priest RE et al. (1978) Origin of cells in human amniotic fluid cultures: ultrastructural features. Lab Invest 39: 106–109
In't Anker PS et al. (2003) Amniotic fluid as a novel source of mesenchymal stem cells for therapeutic transplantation. Blood 102: 1548–1549
Tsai MS et al. (2004) Isolation of human multipotent mesenchymal stem cells from second-trimester amniotic fluid using a novel two-stage culture protocol. Hum Reprod 19: 1450–1456
Prusa AR et al. (2004) Neurogenic cells in human amniotic fluid. Am J Obst Gynecol 191: 309–314
Schmidt D et al. (2007) Prenatally fabricated autologous human living heart valves based on amniotic fluid derived progenitor cells as single cell source. Circulation 116 (Suppl 1): I64–I70
De Coppi P et al. (2007) Isolation of amniotic stem cell lines with potential for therapy. Nat Biotechnol 25: 100–106
French AJ et al. (2008) Development of human cloned blastocysts following somatic cell nuclear transfer with adult fibroblasts. Stem Cells 26: 485–493
Rideout WM III et al. (2001) Nuclear cloning and epigenetic reprogramming of the genome. Science 293: 1093–1098
Rideout WM III et al. (2002) Correction of a genetic defect by nuclear transplantation and combined cell and gene therapy. Cell 109: 17–27
Hochedlinger K and Jaenisch R (2002) Monoclonal mice generated by nuclear transfer from mature B and T donor cells. Nature 415: 1035–1038
Eggan K et al. (2004) Mice cloned from olfactory sensory neurons. Nature 428: 44–49
Hochedlinger K and Jaenisch R (2002) Nuclear transplantation: lessons from frogs and mice. Curr Opin Cell Biol 14: 741–748
Hochedlinger K and Jaenisch R (2003) Nuclear transplantation, embryonic stem cells, and the potential for cell therapy. N Engl J Med 349: 275–286
Simerly C et al. (2003) Molecular correlates of primate nuclear transfer failures. Science 300: 297
Hwang WS et al. (2004) Evidence of a pluripotent human embryonic stem cell line derived from a cloned blastocyst. Science 303: 1669–1674
Hwang WS et al. (2005) Patient-specific embryonic stem cells derived from human SCNT blastocysts. Science 308: 1777–1783
Byrne JA et al. (2007) Producing primate embryonic stem cells by somatic cell nuclear transfer. Nature 450: 497–502
Vajta G (2007) Somatic cell nuclear transfer in its first and second decades: successes, setbacks, paradoxes and perspectives. Reprod Biomed Online 15: 582–590
Chung Y et al. (2006) Embryonic and extraembryonic stem cell lines derived from single mouse blastomeres. Nature 439: 216–219
Zhang X et al. (2006) Derivation of human embryonic stem cells from developing and arrested embryos. Stem Cells 24: 2669–2676
Geber S and Sampaio M (1999) Blastomere development after embryo biopsy: a new model to predict embryo development and to select for transfer. Hum Reprod 14: 782–786
Hardy K (1993) Development of human blastocysts in vitro . In Preimplantation Embryo Development 184–199 (Ed. Bavister BD) New York: Springer–Verlag
Landry DW and Zucker HA et al. (2004) Embryonic death and the creation of human embryonic stem cells. J Clin Invest 114: 1184–1186
Martinez F et al. (2002) Caspase activity in preimplantation human embryos is not associated with apoptosis. Hum Reprod 17: 1584–1590
Hurlbut WB (2005) Altered nuclear transfer as a morally acceptable means for the procurement of human embryonic stem cells. Perspect Biol Med 48: 211–228
Meissner A and Jaenisch R (2006) Generation of nuclear transfer-derived pluripotent ES cells from cloned Cdx2-deficient blastocysts. Nature 439: 212–215
Vauhkonen M et al. (2008) Helicobacter pylori infection induces a reversible expression of the CDX2 transcription factor protein in human gastric epithelium. Scand J Gastroenterol 43: 915–921
Wernig M et al. (2007) In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature 448: 318–324
Takahashi K and Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126: 663–676
Takahashi K et al. (2007) Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131: 861–872
Blelloch R et al. (2006) Reprogramming efficiency following somatic cell nuclear transfer is influenced by the differentiation and methylation state of the donor nucleus. Stem Cells 24: 2007–2013
Lysaght MJ (2002) Maintenance dialysis population dynamics: current trends and long-term implications. J Am Soc Nephrol 13 (Suppl 1): S37–S40
Humes HD et al. (1999) Replacement of renal function in uremic animals with a tissue-engineered kidney. Nat Biotechnol 17: 451–455
MacKay SM et al. (1998) Tissue engineering of a bioartificial renal tubule. ASAIO J 44: 179–183
Aebischer P et al. (1987) The bioartificial kidney: progress towards an ultrafiltration device with renal epithelial cells processing. Life Support Syst 5: 159–168
Ip TK et al. (1988) Cellular control of membrane permeability: implications for a bioartificial renal tubule. ASAIO Trans 34: 351–355
Humes HD (2000) Bioartificial kidney for full renal replacement therapy. Semin Nephrol 20: 71–82
Amiel GE et al. (2000) Renal therapy using tissue-engineered constructs and gene delivery. World J Urol 18: 71–79
Lanza RP et al. (1996) Encapsulated cell technology. Nat Biotechnol 14: 1107–1111
Lanza RP et al. (2002) Generation of histocompatible tissues using nuclear transplantation. Nat Biotechnol 20: 689–696
Humes HD et al. (1996) Tubulogenesis from isolated single cells of adult mammalian kidney: clonal analysis with a recombinant retrovirus. Am J Physiol. Renal Physiol 271: 42–49
Qiao J et al. (1999) Branching morphogenesis independent of mesenchymal-epithelial contact in the developing kidney. Proc Natl Acad Sci USA 96: 7330–7335
Aboushwareb T et al. (2008) Erythropoietin producing cells for potential cell therapy. World J Urol 26: 295–300
Cilento BG et al. (1994) Phenotypic and cytogenetic characterization of human bladder urothelia expanded in vitro . J Urol 152: 665–670
Atala A et al. (2006) Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet 367: 1241–1246
El-Kassaby A et al. (2008) Randomized comparative study between buccal mucosal and acellular bladder matrix grafts in complex anterior urethral strictures. J Urol 179: 1432–1436
El-Kassaby AW et al. (2003) Urethral stricture repair with an off-the-shelf collagen matrix. J Urol 169: 170–173
De Filippo RE et al. (2002) Urethral replacement using cell seeded tubularized collagen matrices. J Urol 168: 1789–1792
Orabi HO et al. (2008) Tissue engineered tubularized urethra for surgical reconstruction: a pre-clinical study [abstract]. FASEB J 22: 581.6
Cannon TW et al. (2003) Improved sphincter contractility after allogenic muscle-derived progenitor cell injection into the denervated rat urethra. Urology 62: 958–963
Peyromaure M et al. (2004) Fate of implanted syngenic muscle precursor cells in striated urethral sphincter of female rats: perspectives for treatment of urinary incontinence. Urology 64: 1037–1041
Lee JY et al. (2004) Long term effects of muscle-derived stem cells on leak point pressure and closing pressure in rats with transected pudendal nerves. Mol Cells 18: 309–313
Mitterberger M et al. (2008) Adult stem cell therapy of female stress urinary incontinence. Eur Urol 53: 169–175
Strasser H et al. (2007) Transurethral ultrasonography-guided injection of adult autologous stem cells versus transurethral endoscopic injection of collagen in treatment of urinary incontinence. World J Urol 25: 385–392
Strasser H et al. (2007) Autologous myoblasts and fibroblasts versus collagen for treatment of stress urinary incontinence in women: a randomised controlled trial. Lancet 369: 2179–2186
Hoshi A et al. (2008) Reconstruction of radical prostatectomy-induced urethral damage using skeletal muscle-derived multipotent stem cells. Transplantation 85: 1617–1624
Mitterberger M et al. (2008) Myoblast and fibroblast therapy for post-prostatectomy urinary incontinence: 1-year followup of 63 patients. J Urol 179: 226–231
Strasser H et al. (2007) Transurethral ultrasound guided injection of autologous myoblasts and fibroblasts in treatment of incontinence after prostate surgery [abstract #1182]. J Urol 177 (Suppl): 390
Kleinert S and Horton R (2008) Retraction—autologous myoblasts and fibroblasts for treatment of stress urinary incontinence: a randomised controlled trial. Lancet 372: 789–790
Bhatt RI et al. (2003) Novel method for the isolation and characterisation of the putative prostatic stem cell. Cytometry A 54: 89–99
Lawson DA et al. (2007) Isolation and functional characterization of murine prostate stem cells. Proc Natl Acad Sci USA 104: 181–186
Lawson DA and Witte ON (2007) Stem cells in prostate cancer initiation and progression. J Clin Invest 117: 2044–2050
Hübner K et al. (2003) Derivation of oocytes from mouse embryonic stem cells. Science 300: 1251–1256
Toyooka Y et al. (2004) Embryonic stem cells can form germ cells in vitro . Proc Natl Acad Sci USA 100: 11457–11462
Kanatsu-Shinohara M and Shinohara T (2007) Culture and genetic modification of mouse germline stem cells. Ann NY Acad Sci 1120: 59–71
Machluf M et al. (2000) Controlled release of therapeutic agents: slow delivery and cell encapsulation. World J Urol 18: 80–83
Machluf M et al. (2003) Microencapsulation of Leydig cells: a system for testosterone supplementation. Endocrinology 144: 4975–4979
Zuk PA et al. (2001) Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng 7: 211–228
Shah NM et al. (1996) Alternative neural crest cell fates are instructively promoted by TGFbeta superfamily members. Cell 85: 331–343
Poulsom R et al. (2001) Bone marrow contributes to renal parenchymal turnover and regeneration. J Pathol 195: 229–235
Brinster RL and Avarbock MR (1994) Germline transmission of donor haplotype following spermatogonial transplantation. Proc Natl Acad Sci USA 91: 11303–11307
Lo KC et al. (2004) De novo testosterone production in luteinizing hormone receptor knockout mice after transplantation of leydig stem cells. Endocrinology 145: 4011–4015
Geijsen N et al. (2004) Derivation of embryonic germ cells and male gametes from embryonic stem cells. Nature 427: 148–154
Strasser H et al. (2004) Stem cell therapy for urinary incontinence [German]. Urologe A 43: 1237–1241
Acknowledgements
The authors wish to thank Dr Jennifer L Olson for editorial assistance with this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
T Aboushwareb has declared that he has conducted research supported by Tengion. A Atala has declared that he has acted as a consultant for and holds stock in Tengion and Plureon, companies involved in developing tissue engineering and stem cell technologies.
Rights and permissions
About this article
Cite this article
Aboushwareb, T., Atala, A. Stem cells in urology. Nat Rev Urol 5, 621–631 (2008). https://doi.org/10.1038/ncpuro1228
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncpuro1228
This article is cited by
-
Towards clinical application of tissue engineering for erectile penile regeneration
Nature Reviews Urology (2019)
-
Tissue-Engineered Urinary Conduits
Current Urology Reports (2015)
-
Stem cell therapy for stress urinary incontinence: a systematic review in human subjects
Archives of Gynecology and Obstetrics (2013)
-
Embryoid body formation of human amniotic fluid stem cells depends on mTOR
Oncogene (2010)