Abstract
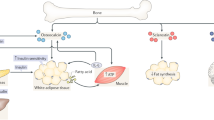
Osteoporosis and obesity, two disorders of body composition, are growing in prevalence. Interestingly, these diseases share several features including a genetic predisposition and a common progenitor cell. With aging, the composition of bone marrow shifts to favor the presence of adipocytes, osteoclast activity increases, and osteoblast function declines, resulting in osteoporosis. Secondary causes of osteoporosis, including diabetes mellitus, glucocorticoids and immobility, are associated with bone-marrow adiposity. In this review, we ask a provocative question: does fat infiltration in the bone marrow cause low bone mass or is it a result of bone loss? Unraveling the interface between bone and fat at a molecular and cellular level is likely to lead to a better understanding of several diseases, and to the development of drugs for both osteoporosis and obesity.
Key Points
-
Bone-marrow stromal cells can differentiate into adipocytes or osteoblasts
-
Bone-marrow adiposity increases with age in mammalian species
-
The function of fat in the bone marrow is unknown; it may be protective or detrimental
-
Increased bone-marrow fat as detected by MRI might be associated with greater fracture risk
-
The master controls over stem-cell lineage allocation are still not well defined
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
US Department of Health and Human Services (2004) Bone Health and Osteoporosis: A Report of the Surgeon General . Rockville: US Department of Health and Human Services
Rosen CJ et al. (2001) Defining the genetics of osteoporosis: using the mouse to understand man. Osteoporos Int 12: 803–810
Recker RR and Heaney RP (1993) Peak bone mineral density in young women. JAMA 270: 2926–2927
Goulding A et al. (2001) Bone mineral density and body composition in boys with distal forearm fractures: a dual-energy X-ray absorptiometry study. J Pediatr 139: 509–515
Brown S and Rosen CJ (2003) Osteoporosis. Med Clin North Am 87: 1039–1063
Kveiborg M et al. (2000) Changes in the insulin-like growth factor-system may contribute to in vitro age-related impaired osteoblast functions. Exp Gerontol 35: 1061–1074
Manolagas SC (2000) Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr Rev 21: 115–137
Horowitz MC and Lorenzo JA (2004) The origins of osteoclasts. Curr Opin Rheumatol 16: 464–468
Gimble JM et al. (1996) The function of adipocytes in the bone marrow stroma: an update. Bone 19: 421–428
Cohen PG (2001) Aromatase, adiposity, aging and disease. The hypogonadal-metabolic-atherogenic-disease and aging connection. Med Hypotheses 56: 702–708
Aubin JE (1998) Bone stem cells. J Cell Biochem Suppl 30–31: 73–82
Ducy P et al. (2000) Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell 100: 197–207
Takeda S et al. (2002) Leptin regulates bone formation via the sympathetic nervous system. Cell 111: 305–317
Elefteriou F et al. (2005) Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature 434: 514–520
Steppan CM et al. (2000) Leptin is a potent stimulator of bone growth in ob/ob mice. Regul Pept 92: 73–78
Cornish J et al. (2002) Leptin directly regulates bone cell function in vitro and reduces bone fragility in vivo. J Endocrinol 175: 405–415
Burguera B. et al. (2001) Leptin reduces ovariectomy-induced bone loss in rats. Endocrinology 142: 3546–3553
Reid IR (2004) Leptin deficiency—lessons in regional differences in the regulation of bone mass. Bone 34: 369–371
Hamrick MW et al. (2004) Leptin deficiency produces contrasting phenotypes in bones of the limb and spine. Bone 34: 376–383
Dhillon H et al. (2004) β-adrenergic receptor KO mice have increased bone mass and strength but are not protected from ovariectomy-induced bone loss [abstract]. J Bone Miner Res 19 (Suppl): S32
Pierroz DD et al. (2004) β1β2 adrenergic receptor KO mice have decreased total body and cortical bone mass despite increased trabecular number [abstract]. J Bone Miner Res 19 (Suppl): S32
Akune T et al. (2004) PPARγ insufficiency enhances osteogenesis through osteoblast formation from bone marrow progenitors. J Clin Invest 113: 846–855
Rzonca SO et al. (2004) Bone is a target for the antidiabetic compound rosiglitazone. Endocrinology 145: 401–406
Moerman EJ et al. (2004) Aging activates adipogenic and suppresses osteogenic programs in mesenchymal marrow stroma/stem cells: the role of PPAR-γ2 transcription factor and TGF-β/BMP signaling pathways. Aging Cell 3: 379–389
Uchiyama Y et al. (1994) Adipose conversion is accelerated in bone marrow cells of congenitally osteoporotic SAMP6 mice [abstract]. J Bone Miner Res 9 (Suppl 1): S321
Botolin S. et al. (2005) Increased bone adiposity and peroxisomal proliferator-activated receptor-γ2 expression in type I diabetic mice. Endocrinology 146: 3622–3631
Rosen CJ et al. (2004) Congenic mice with low serum IGF-I have increased body fat, reduced bone mineral density, and an altered osteoblast differentiation program. Bone 35: 1046–1058
Rosen CJ et al. (2005) Allelic differences in a quantitative trait locus affecting insulin-like growth factor-I impact skeletal acquisition and body composition. Pediatr Nephrol 20: 255–260
Reid IR (2002) Relationships among body mass, its components, and bone. Bone 31: 547–555
Wang MC et al. (2005) The relative contributions of lean tissue mass and fat mass to bone density in young women. Bone 37: 474–481
von Mach MA et al. (2004) Changes in bone mineral content after surgical treatment of morbid obesity. Metabolism 53: 918–921
Cummings SR et al. (1995) Risk factors for hip fracture in white women. Study of Osteoporotic Fractures Research Group. N Engl J Med 332: 767–773
Nguyen ND et al. (online 23 February 2005) Abdominal fat and hip fracture risk in the elderly: the Dubbo Osteoporosis Epidemiology Study. [http://www.biomedcentral.com/1471-2474/6/11] (accessed 4 October 2005)
Garnero P et al. (2000) Biochemical markers of bone turnover, endogenous hormones and the risk of fractures in postmenopausal women: the OFELY study. J Bone Miner Res 15: 1526–1536
Adler RA and Rosen CJ (1994) Glucocorticoids and osteoporosis. Endocrinol Metab Clin North Am 23: 641–654
Hsu Y et al. (2004) Major determinants of bone mineral density (BMD) at multiple skeletal sites in Chinese [abstract]. J Bone Miner Res 19 (Suppl): S421
Wehrli FW et al. (2000) Cross-sectional study of osteopenia with quantitative MR imaging and bone densitometry. Radiology 217: 527–538
Meunier P et al. (1971) Osteoporosis and the replacement of cell populations of the marrow by adipose tissue. A quantitative study of 84 iliac bone biopsies. Clin Orthop Relat Res 80: 147–154
Rozman C et al. (1989) Age-related variations of fat tissue fraction in normal human bone marrow depend both on size and number of adipocytes: a stereological study. Exp Hematol 17: 34–37
Justesen J. et al. (2001) Adipocyte tissue volume in bone marrow is increased with aging and in patients with osteoporosis. Biogerontology 2: 165–171
Verma S et al. (2002) Adipocytic proportion of bone marrow is inversely related to bone formation in osteoporosis. J Clin Pathol 55: 693–698
Schellinger D et al. (2001) Potential value of vertebral proton MR spectroscopy in determining bone weakness. AJNR Am J Neuroradiol 22: 1620–1627
Yeung DK et al. (2005) Osteoporosis is associated with increased marrow fat content and decreased marrow fat unsaturation: a proton MR spectroscopy study. J Magn Reson Imaging 22: 279–285
Tuominen JT et al. (1999) Bone mineral density in patients with type 1 and type 2 diabetes. Diabetes Care 22: 1196–1200
Li X et al. (2005) Steroid effects on osteogenesis through mesenchymal cell gene expression. Osteoporos Int 16: 101–108
Cui Q et al. (2000) Pluripotential marrow cells produce adipocytes when transplanted into steroid-treated mice. Connect Tissue Res 41: 45–56
van Staa TP et al. (2002) The epidemiology of corticosteroid-induced osteoporosis: a meta-analysis. Osteoporos Int 13: 777–787
Ahdjoudj S et al. (2002) Transforming growth factor β2 inhibits adipocyte differentiation induced by skeletal unloading in rat bone marrow stroma. J Bone Miner Res 17: 668–677
Papakitsou EF et al. (2004) Body mass index (BMI) and parameters of bone formation and resorption in postmenopausal women. Maturitas 47: 185–193
Sekiya I et al. (2004) Adipogenic differentiation of human adult stem cells from bone marrow stroma (MSCs). J Bone Miner Res 19: 256–264
Rodriguez JP et al. (2000) Mesenchymal stem cells from osteoporotic patients produce a type I collagen-deficient extracellular matrix favoring adipogenic differentiation. J Cell Biochem 79: 557–565
Weisberg SP et al. (2003) Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 112: 1796–1808
Martin RB and Zissimos SL (1991) Relationships between marrow fat and bone turnover in ovariectomized and intact rats. Bone 12: 123–131
Nuttall ME and Gimble JM (2004) Controlling the balance between osteoblastogenesis and adipogenesis and the consequent therapeutic implications. Curr Opin Pharmacol 4: 290–294
Lecka-Czernik B et al. (1999) Inhibition of Osf2/Cbfa1 expression and terminal osteoblast differentiation by PPARγ2. J Cell Biochem 74: 357–371
Dorheim MA et al. (1993) Osteoblastic gene expression during adipogenesis in hematopoietic supporting murine bone marrow stromal cells. J Cell Physiol 154: 317–328
Beresford JN et al. (1992) Evidence for an inverse relationship between the differentiation of adipocytic and osteogenic cells in rat marrow stromal cell cultures. J Cell Sci 102: 341–351
Acknowledgements
The authors are supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Rosen, C., Bouxsein, M. Mechanisms of Disease: is osteoporosis the obesity of bone?. Nat Rev Rheumatol 2, 35–43 (2006). https://doi.org/10.1038/ncprheum0070
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ncprheum0070
This article is cited by
-
Association of visceral and subcutaneous fat with bone mineral density in US adults: a cross-sectional study
Scientific Reports (2023)
-
Nutrient regulation of bone marrow adipose tissue: skeletal implications of weight loss
Nature Reviews Endocrinology (2023)
-
CT and MR for bone mineral density and trabecular bone score assessment in osteoporosis evaluation
Scientific Reports (2023)
-
Bone mineral density and lipid profiles in older adults: a nationwide cross-sectional study
Osteoporosis International (2023)
-
Adipopenia is associated with osteoporosis in community-dwelling non-underweight adults independent of sarcopenia
Archives of Osteoporosis (2023)