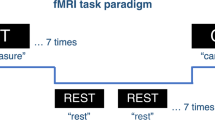
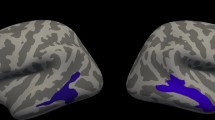
Abstract
Functional MRI (fMRI) might provide important insights into emerging data that suggest that recovery from injury can occur in the brains of children born prematurely. Strategies employing auditory stimulation demonstrate blood-oxygen-level-dependent (BOLD) activation in preterm infants as young as 33 weeks' gestational age, and reliable BOLD signal in response to visual stimulation occurs at term-equivalent age. Strategies based on fMRI are particularly suited to the study of language and memory, and emerging data are likely to provide insights into perplexing reports that have demonstrated improving cognitive scores but persistent volumetric and microstructural changes in frontotemporal language systems in the prematurely born. Even when sex, gestational age and early medical and environmental interventions are taken into account, fMRI data from several investigators suggest the engagement of alternative neural networks for language and memory in the developing preterm brain.
Key Points
-
Functional MRI (fMRI) might provide insight into the neurobiological mechanisms supporting recovery from injury in prematurely born individuals
-
fMRI can detect activation to auditory stimuli in preterm neonates as early as 33 weeks' postmenstrual age; blood-oxygen-level-dependent signal to visual stimulation can be reliably detected in preterm infants at term-equivalent age
-
fMRI investigations of language suggest the engagement of alternative compensatory neural systems in preterm children at school age and beyond
-
Gestational age, sex and structural and microstructural cerebral changes must be considered when evaluating fMRI tasks in the prematurely born population
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Callaghan WM et al. (2006) The contribution of preterm birth to infant mortality rates in the United States. Pediatrics 118: 1566–1573
Hack M et al. (2005) Poor predictive validity of the Bayley Scales of Infant Development for cognitive function of extremely low birth weight children at school age. Pediatrics 116: 333–341
Hack MB et al. (2002) Outcomes in young adulthood for very-low-birth-weight infants. N Engl J Med 346: 149–157
Roth S et al. (2001) Neurodevelopmental status at 1 year predicts neuropsychiatric outcome at 14–15 years of age in very preterm infants. Early Hum Dev 65: 81–89
Ment LR et al. (2003) Change in cognitive function over time in very low-birth-weight infants. JAMA 289: 705–711
Perlman JM and Rollins N (2000) Surveillance protocol for the detection of intracranial abnormalities in premature neonates. Arch Pediatr Adolesc Med 154: 822–826
de Vries LS and Groenendaal F (2002) Neuroimaging in the preterm brain. Ment Retard Dev Disabil Res Rev 8: 273–280
Woodward LJ et al. (2006) Neonatal MRI to predict neurodevelopmental outcomes in preterm infants. N Engl J Med 355: 685–694
Dyet LE et al. (2006) Natural history of brain lesions in extremely preterm infants studied with serial magnetic resonance imaging from birth and neurodevelopmental assessment. Pediatrics 118: 536–548
Johnston MV et al. (2001) Sculpting the developing brain. Adv Pediatr 48: 1–38
Vaccarino FM et al. (2001) Stem cells in neurodevelopment and plasticity. Neuropsychopharmacology 25: 806–815
Faverjon S et al. (2002) Beneficial effects of enriched environment following status epilepticus in immature rats. Neurology 59: 1356–1364
van Praag H et al. (1999) Running enhances neurogenesis, learning and long-term potentiation in mice. Proc Natl Acad Sci USA 96: 13427–13431
Alvarez-Buylla A et al. (2000) The subventricular zone: source of neuronal precursors for brain repair. Prog Brain Res 127: 1–11
Stewart AL et al. (1999) Brain structure and neurocognitive and behavioural function in adolescents who were born very preterm. Lancet 353: 1653–1657
Curtis WJ et al. (2006) Memory in early adolescents born prematurely: a functional magnetic resonance imaging investigation. Dev Neuropsychol 29: 341–377
Yu V (2000) Developmental outcomes of extremely preterm infants. Am J Perinatol 2: 57–61
Saigal S et al. (2006) Transition of extremely low-birth-weight infants from adolescence to young adulthood. JAMA 295: 667–675
Nosarti C et al. (2002) Adolescents who were born very preterm have decreased brain volumes. Brain 125: 1616–1623
Reiss AL et al. (2004) Sex differences in cerebral volumes of 8-year-olds born preterm. J Pediatr 145: 242–249
Lodygensky GA et al. (2005) Structural and functional brain development after hydrocortisone treatment for neonatal chronic lung disease. Pediatrics 116: 1–7
Gimenez M et al. (2006) White matter volume and concentration reductions in adolescents with history of very preterm birth: a voxel-based morphometry study. Neuroimage 32: 1485–1498
Gimenez M et al. (2006) Abnormal orbitofrontal development due to prematurity. Neurology 67: 1818–1822
Rushe TM et al. (2001) Neuropsychological outcome at adolescence of very preterm birth and its relation to brain structure. Dev Med Child Neurol 43: 226–233
Peterson BS et al. (2000) Regional brain volume abnormalities and long-term cognitive outcome in preterm infants. JAMA 284: 1939–1947
Kesler SR et al. (2004) Volumetric analysis of regional cerebral development in preterm children. Pediatr Neurol 31: 318–325
Gimenez M et al. (2004) Hippocampal gray matter reduction associated with memory deficits in adolescents with history of prematurity. Neuroimage 23: 869–877
Mukherjee P and McKinstry RC (2006) Diffusion tensor imaging and tractography of human brain development. Neuroimag Clin N Am 16: 19–43
Vangberg TR et al. (2006) Changes in white matter diffusion anisotropy in adolescents born prematurely. Neuroimage 32: 1538–1548
Skranes JS et al. (2007) Clinical findings and white matter abnormalities seen on diffusion tensor imaging in adolescents with very low birth weight. Brain 130: 654–666
Ravnkilde B et al. (2002) Putative tests of frontal lobe function: a PET study of brain activation during Stroop's test and verbal fluency. J Clin Exp Neuropsychol 24: 534–547
Annoni JM et al. (2003) Chronic cognitive impairment following laterothalamic infarcts: a study of 9 cases. Arch Neurol 60: 1439–1443
Crosson B (1999) Subcortical mechanisms in language: lexical–semantic mechanisms and the thalamus. Brain Cogn 40: 414–438
Gimenez M et al. (2006) Correlations of thalamic reductions with verbal fluency impairment in those born prematurely. Neuroreport 17: 463–466
Nosarti C et al. (2004) Corpus callosum size and very preterm birth: relationship to neuropsychological outcome. Brain 127: 2080–2089
Fox P and Raichle M (1986) Focal physiological uncoupling of cerebral blood flow and oxidative metabolism during somatosensory stimulation in human subjects. Proc Natl Acad Sci USA 83: 1140–1144
Logothetis NK et al. (2001) Neurophysiological investigation of the basis of the fMRI signal. Nature 412: 150–157
Belliveau JW et al. (1991) Functional mapping of the human visual cortex by magnetic resonance imaging. Science 254: 716–719
Lu H et al. (2003) Functional magnetic resonance imaging based on changes in vascular space occupancy. Magn Reson Med 50: 263–274
Davis TL et al. (1998) Calibrated functional MRI: mapping the dynamics of oxidative metabolism. Proc Natl Acad Sci USA 95: 1834–1839
Born P et al. (1998) Visual activation in infants and young children studied by functional magnetic resonance imaging. Pediatr Res 44: 578–583
Born AP et al. (2000) Functional magnetic resonance imaging of the normal and abnormal visual system in early life. Neuropediatrics 31: 24–32
Erberich SG et al. (2003) Functional MRI in neonates using neonatal head coil and MR compatible incubator. Neuroimage 20: 683–692
Anderson AW et al. (2001) Neonatal auditory activation detected by functional magnetic resonance imaging. Magn Reson Imaging 19: 1–5
Erberich SG et al. (2006) Somatosensory lateralization in the newborn brain. Neuroimage 29: 155–161
Santhouse AM et al. (2002) The functional significance of perinatal corpus callosum damage: an fMRI study in young adults. Brain 125: 1782–1792
Shaywitz BA et al. (2006) The role of functional magnetic resonance imaging in understanding reading and dyslexia. Dev Neuropsychol 30: 613–632
Peterson BS et al. (2002) A functional MRI study of language processing and cognitive outcome in prematurely born children. Pediatrics 110: 1153–1162
Ment LR et al. (2006) Cortical recruitment patterns in children born prematurely compared with control children druing a passive listening functional magnetic resonance imaging task. J Pediatr 149: 490–498
Rushe TM et al. (2004) Lateralisation of language function in young adults born very preterm. Arch Dis Child Fetal Neonatal Ed 89: F112–F118
Maguire EA et al. (2001) The effects of bilateral hippocampal damage on fMRI regional activations and interactions during memory retrieval. Brain 124: 1156–1170
Gimenez M et al. (2005) Hippocampal functional magnetic resonance imaging during a face-name learning task in adolescents with antecedents of prematurity. Neuroimage 25: 561–569
Carmody DP et al. (2006) Early risk, attention and brain activation in adolescents born preterm. Child Dev 77: 384–394
Nosarti C et al. (2006) Altered functional neuroanatomy of reponse inhibition in adolescent males who were born very preterm. Dev Med Child Neurol 48: 265–271
Rivkin MJ et al. (2004) Prolonged T*2 values in newborn versus adult brain: implications for fMRI studies of newborns. Magn Reson Med 51: 1287–1291
Almi CR et al. (2007) The NIH MRI study of normal brain development (Objective-2): newborns, infants, toddlers, and preschoolers. Neuroimage 35: 308–325
Yamada H et al. (2000) A milestone for normal development of the infantile brain detected by functional MRI. Neurology 55: 218–223
Marcar VL et al. (2006) How depth of anesthesia influences the blood oxygenation level-dependent signal from the visual cortex of children. AJNR Am J Neuroradiol 27: 799–805
Dueck MH et al. (2005) Propofol attenuates responses of the auditory cortex to acoustic stimulation in a dose-dependent manner: a fMRI study. Acta Anaesthesiol Scand 49: 488–457
Heinke W and Koelsch S (2005) The effects of anesthetics on brain activity and cognitive function. Curr Opin Anesthesiol 18: 625–631
Kerssens C et al. (2005) Attenuated brain response to auditory word stimulation with sevoflurane: a functional magnetic resonance study in humans. Anesthesiology 103: 11–19
Plourde G et al. (2006) Cortical processing of complex auditory stimuli during alterations of consciousness with the general anesthetic propofol. Anesthesiology 104: 448–457
Ewers M et al. (2006) Multicenter assessment of reliability of cranial MRI. Neurobiol Aging 27: 1051–1059
Schnack HG et al. (2004) Reliability of brain volumes from multicenter MRI acquisition: a calibration study. Hum Brain Mapp 22: 312–320
Friedman L et al. (2006) Reducing interscanner variability of activation in a multi-center fMRI study: controlling for signal-to-fluctuation-noise-ratio (SFNR) differences. Neuroimage 33: 471–481
Pfefferbaum A et al. (2003) Replicability of diffusion tensor imaging measurements of fractional anisotropy and trace in brain. J Magn Reson Imaging 28: 427–433
Scouten A et al. (2006) Spatial resolution, signal-to-noise ratio and smoothing in multi-subject functional MRI studies. Neuroimage 30: 787–793
Friedman L and Glover GH (2006) Report on a multicenter fMRI quality assurance protocol. J Magn Reson Imaging 23: 827–839
Zou KH et al. (2005) Reproducibility of functional MR imaging: preliminary results of prospective multi-institutional study performed by Biomedical Informatics Research Network. Radiology 237: 781–789
Martin E et al. (1999) Visual processing in infants and children studied using functional MRI. Pediatr Res 46: 135–140
Born AP et al. (2002) Visual cortex reactivity in sedated children examined with perfusion MRI (FAIR). Magn Reson Imaging 20: 199–205
Meek JH et al. (1998) Regional hemodynamic responses to visual stimulation in awake infants. Pediatr Res 43: 840–843
Shmuel A et al. (2006) Negative functional MRI response correlates with decreases in neuronal activity in monkey visual area V1. Nat Neurosci 9: 569–577
Allison JD et al. (2000) Functional MRI cerebral activation and deactivation during finger movement. Neurology 55: 135–142
Raichle ME et al. (2001) A default mode of brain function. Proc Natl Acad Sci USA 98: 676–682
Smith AT et al. (2004) Negative BOLD in the visual cortex: evidence against blood stealing. Hum Brain Mapp 21: 213–220
Harel N et al. (2002) Origin of negative blood oxygenation level-dependent fMRI signals. J Cereb Blood Flow Metab 22: 908–917
Tootell RB et al. (1998) The representation of the ipsilateral visual field in human cerebral cortex. Proc Natl Acad Sci USA 95: 818–824
Nowinski WL and Thirunavuukarasuu A (2001) Atlas-assisted localization analysis of functional images. Med Image Anal 25: 207–220
Lancaster JL et al. (2000) Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp 20: 120–131
Burgund ED et al. (2002) The feasibility of a common stereotactic space for children and adults in fMRI studies of development. Neuroimage 17: 184–200
Kang HC et al. (2003) Comparison of functional activation foci in children and adults using a common stereotactic space. Neuroimage 19: 16–28
Narberhaus A et al. (2007) Gestational age at preterm birth in relation to corpus callosum and general cognitive outcome in adolescents. J Child Neurol 22: 761–765
Huppi PS et al. (1998) Microstructural development of human newborn cerebral white matter assessed in vivo by diffusion tensor magnetic resonance imaging. Pediatr Res 44: 584–590
Neil JJ et al. (1998) Normal brain in human newborns: apparent diffusion coefficient and diffusion anisotropy measured by using diffusion tensor imaging. Radiology 209: 57–66
Ment LR et al. (2006) A functional MRI study of the long-term influences of early indomethacin exposure on language processing in the brains of prematurely born children. Pediatrics 118: 961–970
Constable RT et al.: Prematurely born children demonstrate white matter microstructural differences at age 12 years relative to term controls: an investigation of group and gender effects. Pediatrics, in press
Sowell ER et al. (2006) Sex differences in cortical thickness mapped in 176 healthy individuals between 7 and 87 years of age. Cereb Cortex 17: 1550–1560
Sowell ER et al. (2004) Longitudinal mapping of cortical thickness and brain growth in normal children. J Neurosci 24: 8223–8231
Als H et al. (2004) Early experience alters brain function and structure. Pediatrics 114: 1738–1739
Nobre AC et al. (2003) Brain activations during visual search: contributions of search efficiency versus feature binding. Neuroimage 18: 91–103
Meltzer JA and Constable RT (2005) Activation of human hippocampal formation reflects success in both encoding and cued recall of paired associates. Neuroimage 24: 384–397
Toni I et al. (2002) Changes of cortico-striatal effective connectivity during visuomotor learning. Cereb Cortex 12: 1040–1047
Gauthier I et al. (1999) Activation of the middle fusiform face area increases with expertise in recognizing novel objects. Nat Neurosci 2: 568–573
Grossman M et al. (2002) The neural basis for categorization in semantic memory. Neuroimage 17: 1549–1561
Grady CL et al. (2001) An examination of the effects of stimulus type, encoding task and functional connectivity on the role of right prefrontal cortex in recognition memory. Neuroimage 24: 556–571
Saigal S et al. (2003) Psychopathology and social competencies of adolescents who were extremely low birth weight. Pediatrics 111: 969–975
Saigal S et al. (2003) School-age outcomes in children who were extremely low birth weight from four international population-based cohorts. Pediatrics 112: 943–950
Ment LR et al. (2005) Grade 3 to 4 intraventricular hemorrhage and Bayley scores predict outcome. Pediatrics 116: 1597–1598
Dobbing J and Sands J (1973) Quantitative growth and development of human brain. Arch Dis Child 48: 757–767
Huttenlocher PR (1979) Synaptic density in human frontal cortex—developmental changes and effects of aging. Brain Res 163: 195–205
Huttenlocher P and Dabholkar A (1997) Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol 387: 167–178
Blumenfeld HK et al. (2006) Differential prefrontal–temporal neural correlates of semantic processing in children. Brain Lang 99: 226–235
Brown TB et al. (2005) Developmental changes in human cerebral functional organization for word generation. Cereb Cortex 15: 275–290
Chou TL et al. (2006) Developmental changes in the neural correlates of semantic processing. Neuroimage 29: 1141–1149
Brizzolara D et al. (2002) Timing and type of congenital brain lesion determine different patterns of language lateralization in hemiplegic children. Neuropsychologia 40: 620–632
Shaywitz BA et al. (2004) Development of left occipitotemporal systems for skilled reading in children after a phonologically-based intervention. Biol Psychiatry 55: 926–933
Sporns O et al. (2005) The human connectome: a structural description of the human brain. PLoS Comput Biol 1: 245–251
Leonard CM et al. (2006) Exploiting human anatomical variability as a link between genome and cognome. Genes Brain Behav 5: 64–77
Acknowledgements
The authors wish to thank Drs Deborah Hirtz, Betty Vohr, Walter Allan, Kenneth Pugh, Robin Schafer and Allan Reiss for scientific advice; they also thank Ms Cheryl Lacadie, Karol Katz and Karen Schneider for scientific and technical support. The authors' work was supported by NIH/NINDS grants NS27116, NS35476, NS038467, NS047605 and NS051622.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Ment, L., Constable, R. Injury and recovery in the developing brain: evidence from functional MRI studies of prematurely born children. Nat Rev Neurol 3, 558–571 (2007). https://doi.org/10.1038/ncpneuro0616
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ncpneuro0616
This article is cited by
-
Trajectories of brain development in school-age children born preterm with very low birth weight
Scientific Reports (2018)
-
A crucial role for white matter alterations in interference control problems of very preterm children
Pediatric Research (2014)