Abstract
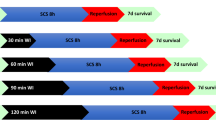
There is a persistent shortage of kidneys available for transplantation. In the early 1980s, therefore, we published the concept of non-heartbeating (NHB) donation; that is, procurement of kidneys from donors whose death has been accompanied by irreversible circulatory arrest. NHB donors are generally categorized using four definitions; category III (awaiting cardiac arrest) and category IV (cardiac arrest while braindead)—or 'controlled'—donors are the most suitable for initiating NHB donation programs. Delayed graft function is associated with use of kidneys from such donors, but has no effect on graft survival in the short or long term. Use of kidneys from category I (dead upon arrival at hospital) and category II (unsuccessfully resuscitated), or 'uncontrolled', donors is likewise associated with delayed graft function, but also with an increased risk of primary nonfunction. Viability testing of donated organs from these sources is a prerequisite for transplantation. Machine preservation parameters and enzyme release measurements help to distinguish viable from nonviable kidneys. The proportion of NHB donor kidneys in the total pool of postmortem kidneys differs considerably between countries. In The Netherlands, the proportion is nearly 50%. This figure is markedly higher than that in the US and Canada, where national programs have now been initiated to increase rates of NHB donation. In the future, warm preservation techniques might facilitate better viability testing, thereby increasing NHB donation from category I and II donors and further reducing the shortage of kidneys available for transplantation.
Key Points
-
Use of more kidneys from non-heartbeating (NHB) donors has the potential to markedly decrease transplantation waiting times
-
Renal allografts from NHB donors are generally more likely to undergo a period of delayed function than those from heartbeating donors
-
Short-term and long-term survival of kidney grafts from NHB donors is equivalent to that of organs from heartbeating donors
-
The frequency of utilization of kidneys from NHB donors varies considerably between and within countries
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
[No authors listed] (1968) A definition of irreversible coma: Report of the Ad Hoc Committee of the Harvard Medical School to Examine the Definition of Brain Death. JAMA 205: 337–340
van der Vliet JA et al. (1980) Non-heartbeating donors; is it worthwhile? Proc Eur Dial Transplant Assoc 17: 445–449
Koffman CG et al. (1993) Comparative study of the use of systolic and asystolic kidney donors between 1988 and 1991. The South Thames Transplant Group. Transplant Proc 25: 1527–1529
Kootstra G et al. (1995) Categories of non-heart-beating donors. Transplant Proc 27: 2893–2894
Garcia-Rinaldi R et al. (1975) In situ preservation of cadaver kidneys for transplantation: laboratory observations and clinical application. Ann Surg 182: 576–584
Wilson CH et al. (2004) Evaluation of eight preservation solutions for endothelial in situ preservation. Transplantation 78: 1008–1013
Herdman R et al. (1998) The Institute of Medicine's report on non-heart-beating organ transplantation. Kennedy Inst Ethics J 8: 83–90
Wijnen RM et al. (1995) Outcome of transplantation of non-heart-beating donor kidneys. Lancet 345: 1067–1070
Metcalfe MS et al. (2001) A case-control comparison of the results of renal transplantation from heart-beating and non-heart-beating donors. Transplantation 71: 1556–1559
Weber M et al. (2002) Kidney transplantation from donors without a heartbeat. N Engl J Med 347: 248–255
Gok MA et al. (2002) Long-term renal function in kidneys from non-heart-beating donors: a single-center experience. Transplantation 74: 664–669
Sudhindran S et al. (2003) Outcome of transplantation using kidneys from controlled (Maastricht category 3) non-heart-beating donors. Clin Transplant 17: 93–100
Chapman J et al. (2006) Follow-up after renal transplantation with organs from donors after cardiac death. Transpl Int 19: 715–719
Cho YW et al. (1998) Transplantation of kidneys from donors whose hearts have stopped beating. N Engl J Med 338: 221–225
Nicholson ML et al. (2000) A comparison of the results of renal transplantation from non-heart-beating, conventional cadaveric, and living donors. Kidney Int 58: 2585–2591
Keizer KM et al. (2005) Non-heart-beating donor kidneys in the Netherlands: allocation and outcome of transplantation. Transplantation 79: 1195–1199
Sanchez-Fructuoso A et al. (2004) Non-heart beating donors. Nephrol Dial Transplant 19 (Suppl 3): Siii26–Siii31
Alonso A et al. (2005) Renal transplantation from non-heart-beating donors: a single-center 10-year experience. Transplant Proc 37: 3658–3660
Cooper JT et al. (2004) Donation after cardiac death: the University of Wisconsin experience with renal transplantation. Am J Transplant 4: 1490–1494
Lee CY et al. (2005) Expanding the donor pool: use of renal transplants from non-heart-beating donors supported with extracorporeal membrane oxygenation. Clin Transplant 19: 383–390
Tanabe K et al. (1998) Long-term renal function in on-heart-beating donor kidney transplantation: a single-center experience. Transplantation 66: 1708–1713
Light JA et al. (1999) Long-term graft survival after transplantation with kidneys from uncontrolled nonheartbeating donors. Transplantation 68: 1910–1911
Alvarez J et al. (2000) Non-heart-beating donors from the streets: an increasing donor pool source. Transplantation 70: 314–317
Hattori R et al. (2003) Long-term outcome of kidney transplant using non-heart-beating donor: multicenter analysis of factors affecting graft survival. Clin Transplant 17: 518–521
de Boer J et al. (1999) Eurotransplant randomized multicenter kidney graft preservation study comparing HTK with UW and Euro-Collins. Transpl Int 12: 447–453
Ploeg RJ et al. (1992) Effect of preservation solution on results of cadaveric kidney transplantation. The European Multicentre Study Group. Lancet 340: 129–137
Booster MH et al. (1994) University of Wisconsin solution is superior to histidine tryptophan ketoglutarate for preservation of ischemically damaged kidneys. Transplantation 58: 979–984
Roels L et al. (1998) Inferior outcome of cadaveric kidneys preserved for more than 24 hr in histidine-tryptophan-ketoglutarate solution. Leuven Collaborative Group for Transplantation. Transplantation 66: 1660–1664
Schmitz V et al. (2006) Impact of organ preservation using HTK for graft flush and subsequent storage in UW in rat kidney transplantation. Eur Surg Res 38: 388–398
Balupuri S et al. (2000) The trouble with kidneys derived from the non heart-beating donor: a single center 10-year experience. Transplantation 69: 842–846
St Peter SD et al. (2002) Liver and kidney preservation by perfusion. Lancet 359: 604–613
Wight JP et al. (2003) Pulsatile machine perfusion vs. cold storage of kidneys for transplantation: a rapid and systematic review. Clin Transplant 17: 293–307
Kozaki K et al. (2000) Prediction of kidney nonfunction after transplantation with machine perfusion preservation. Transplant Proc 32: 275–276
Wilson CH et al. (2004) Weight increase during machine perfusion may be an indicator of organ and in particular, vascular damage. Ann Transplant 9: 31–32
Daemen JH et al. (1995) Viability assessment of non-heart-beating donor kidneys during machine preservation. Transplant Proc 27: 2906–2907
Daemen JW et al. (1997) Glutathione S-transferase as predictor of functional outcome in transplantation of machine-preserved non-heart-beating donor kidneys. Transplantation 63: 89–93
Kievit JK et al. (1997) Release of alpha-glutathione S-transferase (alpha GST) and pi-glutathione S-transferase (pi GST) from ischemic damaged kidneys into the machine perfusate—relevance to viability assessment. Transplant Proc 29: 3591–3593
Van Kreel BK et al. (2002) Functional relationship of alpha-glutathione S-transferase and glutathione S-transferase activity in machine-preserved non-heart-beating donor kidneys. Transpl Int 15: 546–549
Gok MA et al. (2003) Use of two biomarkers of renal ischemia to assess machine-perfused non-heart-beating donor kidneys. Clin Chem 49: 172–175
de Vries B et al. (2004) Reduction of circulating redox-active iron by apotransferrin protects against renal ischemia-reperfusion injury. Transplantation 77: 669–675
de Vries B et al. (2006) Redox-active iron released during machine perfusion predicts viability of ischemically injured deceased donor kidneys. Am J Transplant 6: 2686–2693
Snoeijs MG et al. (2006) Kidney transplantation using elderly non-heart-beating donors: a single-center experience. Am J Transplant 6: 1066–1071
Ojo AO et al. (1997) Delayed graft function: risk factors and implications for renal allograft survival. Transplantation 63: 968–974
Shoskes DA and Cecka JM (1997) Effect of delayed graft function on short- and long-term kidney graft survival. Clin Transpl 297–303
McLaren AJ et al. (1999) Delayed graft function: risk factors and the relative effects of early function and acute rejection on long-term survival in cadaveric renal transplantation. Clin Transplant 13: 266–272
Gjertson DW (2000) Impact of delayed graft function and acute rejection on kidney graft survival. Clin Transpl 467–480
Brook NR et al. (2003) Non-heart beating donor kidneys with delayed graft function have superior graft survival compared with conventional heart-beating donor kidneys that develop delayed graft function. Am J Transplant 3: 614–618
Bains JC et al. (2005) Comparison of renal allograft fibrosis after transplantation from heart-beating and non-heart-beating donors. Br J Surg 92: 113–118
Gerstenkorn C et al. (2002) Outcome of renal allografts from non-heart-beating donors with delayed graft function. Transpl Int 15: 660–663
Renkens JJ et al. (2005) Outcome of nonheart-beating donor kidneys with prolonged delayed graft function after transplantation. Am J Transplant 5: 2704–2709
Gomez M et al. (1993) Cardiopulmonary bypass and profound hypothermia as a means for obtaining kidney grafts from irreversible cardiac arrest donors: cooling technique. Transplant Proc 25: 1501–1502
Sanchez-Fructuoso AI et al. (2000) Renal transplantation from non-heart beating donors: a promising alternative to enlarge the donor pool. J Am Soc Nephrol 11: 350–358
Ko WJ et al. (2000) Extracorporeal membrane oxygenation support of donor abdominal organs in non-heart-beating donors. Clin Transplant 14: 152–156
Magliocca JF et al. (2005) Extracorporeal support for organ donation after cardiac death effectively expands the donor pool. J Trauma 58: 1095–1101
Gok MA et al. (2003) How to improve the quality of kidneys from non-heart-beating donors: a randomised controlled trial of thrombolysis in non-heart-beating donors. Transplantation 76: 1714–1719
Mi H et al. (2005) Do recipients of kidneys from donors treated with streptokinase develop anti-streptokinase antibodies? Transplant Proc 37: 3272–3273
Grinyo JM et al. (2003) Calcineurin inhibitor-free immunosuppression based on antithymocyte globulin and mycophenolate mofetil in cadaveric kidney transplantation: results after 5 years. Transpl Int 16: 820–827
Sonoda T et al. (2003) Outcome of 3 years of immunosuppression with tacrolimus in more than 1,000 renal transplant recipients in Japan. Transplantation 75: 199–204
Wilson CH et al. (2005) Randomized clinical trial of daclizumab induction and delayed introduction of tacrolimus for recipients of non-heart-beating kidney transplants. Br J Surg 92: 681–687
Shemie SD et al. (2006) Organ donor management in Canada: recommendations of the forum on Medical Management to Optimize Donor Organ Potential. CMAJ 174: S13–S32
Bernat JL et al. (2006) Report of a National Conference on Donation after cardiac death. Am J Transplant 6: 281–291
D'Alessandro AM et al. (2004) Donation after cardiac death: the University of Wisconsin experience. Ann Transplant 9: 68–71
Merion RM et al. (2005) Deceased-donor characteristics and the survival benefit of kidney transplantation. JAMA 294: 2726–2733
Nicholson ML et al. (1996) Work-load generated by the establishment of a non-heart beating kidney transplant programme. Transpl Int 9: 603–606
Brasile L et al. (2002) Overcoming severe renal ischemia: the role of ex vivo warm perfusion. Transplantation 73: 897–901
Terasaki PI et al. (1997) Strategy for eliminating the kidney shortage. Clin Transpl 265–267
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Kootstra, G., van Heurn, E. Non-heartbeating donation of kidneys for transplantation. Nat Rev Nephrol 3, 154–163 (2007). https://doi.org/10.1038/ncpneph0426
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ncpneph0426
This article is cited by
-
Machine perfusion versus cold storage of livers: a meta-analysis
Frontiers of Medicine (2016)
-
The use of personalized medicine for patient selection for renal transplantation: Physicians' views on the clinical and ethical implications
BMC Medical Ethics (2010)
-
Kidney transplantation and donation in children
Pediatric Surgery International (2009)