Abstract
The palladium-catalysed allylic substitution reaction is one of the most important reactions in transition-metal catalysis and has been well-studied in the past decades. Most of the reactions proceed through an outer-sphere mechanism, affording linear products when monosubstituted allyl reagents are used. Here, we report an efficient Palladium-catalysed protocol for reactions of β-substituted ketones with monosubstituted allyl substrates, simply by using N-heterocyclic carbene as ligand, leading to branched products with up to three contiguous stereocentres in a (syn, anti)-mode with excellent regio and diastereoselectivities. The scope of the protocol in organic synthesis has been examined preliminarily. Mechanistic studies by both experiments and density functional theory (DFT) calculations reveal that the reaction proceeds via an inner-sphere mechanism—nucleophilic attack of enolate oxygen on Palladium followed by C–C bond-forming [3,3’]-reductive elimination.
Similar content being viewed by others
Introduction
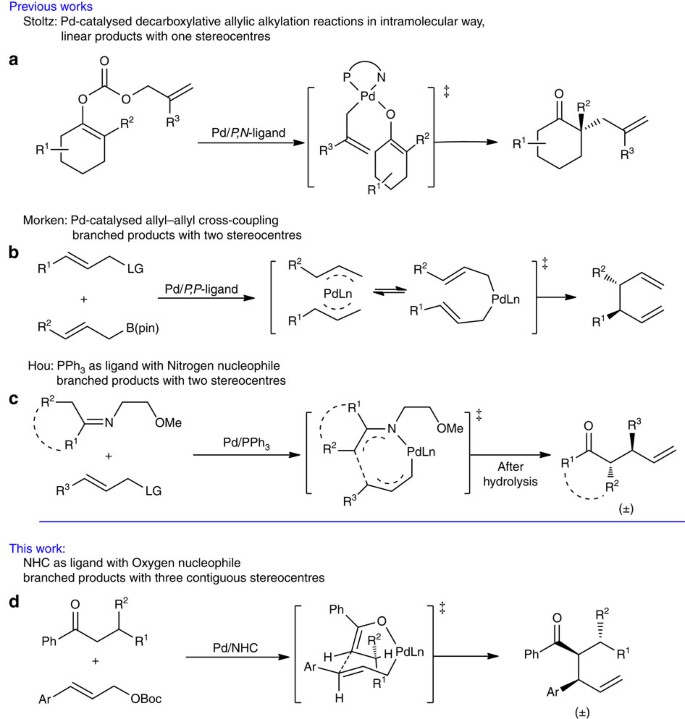
The palladium-catalysed allylic substitution reaction of allyl reagents with nucleophiles has become one of the most important carbon–carbon and carbon-hetero-atom bond-forming processes1,2,3,4. In most cases, the reaction proceeds through an outer-sphere mechanism, in which the nucleophile attacks the allyl carbon of π-allyl-Pd complex, and affords linear products when monosubstituted allyl reagents are used1,2,3,4,5,6,7,8,9,10,11,12. Less commonly, the reaction can follow an inner-sphere mechanism; in this case, the nucleophilic attack is targeted at Pd, forming an intermediate at first, followed by reductive elimination13,14,15,16,17,18,19,20. Recently, several studies on Pd-catalysed allylic substitution/coupling reactions via inner-sphere mechanism have been reported21,22,23,24,25,26,27,28,29,30,31,32,33. The key C–C bond-forming step was proposed to occur via [3,3’]-reductive elimination in an allyl-Pd-allyl or allyl-Pd-nucleophile intermediate. For example, Stoltz27 achieved the construction of a chiral quaternary carbon centre at the α-position of cyclic ketones using unsubstituted allyl carbonates in an intramolecular decarboxylative process (Fig. 1a)25,26,27. Morken realized excellent regio- and enantio-selectivities in the cross-coupling of monosubstituted allyl reagents with allyl boronates under Pd catalysis (Fig. 1b)28,29,30,31,32. These results reveal the unique character of the inner-sphere mechanism; in particular, branched products can be formed as major products when monosubstituted allyl reagents are used21,28. Pd-catalysed allylic substitution via an inner-sphere mechanism also found applications in organic synthesis26,32. However, so far only limited reaction modes following an inner-sphere process have been explored, that is, coupling of diallyl-Pd-species21,28,29,30,31 and an intramolecular decarboxylative process, with narrow substrate scope for the latter (Fig. 1a)27,34. The development of more reaction modes with wider substrate scope remains a great challenge.
(a) Pd-Catalysed intramolecular decarboxylative allylic alkylation. (b) Pd-catalysed allyl–allyl cross-coupling reaction, branched products with two stereocentres were given. (c) Pd-catalysed allylic alkylation with nitrogen as nucleophile affording branched products with two stereocentres. (d) Pd-catalysed allylic alkylation with oxygen as nucleophile affording branched products with three contiguous stereocentres.
Recently, we developed a regio- and chemo-tunable Pd-catalysed allylic alkylation with nitrogen nucleophiles, yielding branched allylated products with excellent regioselectivity; mechanistic investigations including DFT calculations support an inner-sphere mechanism for the reaction (Fig. 1c)33. In further investigations, we realized (with an oxygen nucleophile) Pd-catalysed allylic alkylation of monosubstituted allyl reagents by acyclic ketones. Acyclic compounds having up to three contiguous stereocentres can be obtained with excellent regio and diastereoselectivities (Fig. 1d). The key to high selectivity is the use of an N-heterocyclic carbene (NHC) as ligand. In this paper, we would like to report this new type of Pd/NHC-catalysed allylic alkylation (Fig. 1d) as well as mechanistic studies on the reaction by both experiments and DFT calculations. The scope of the protocol in organic synthesis is also examined preliminarily.
Results
Reaction design
To test whether oxygen nucleophiles are also compatible, as nitrogen nucleophiles are, in the Pd/PPh3-catalysed allylic substitution to give branched products via an inner-sphere mechanism33, we began our investigations with the reaction of propiophenone 1a with cinnamyl tert-butyl carbonate 2a, using [Pd(η3-C3H5)Cl]2 and PPh3 as catalyst and t-BuOK or lithium diisopropylamide as base. Unfortunately, the linear product was obtained as the major component in both cases, with branched to linear ratios (B/L) of 6/94 and 15/85, respectively, despite high yields (95% and 98%). Considering the similar coordination chemistry of NHC and phosphine ligands but different stereochemistry of reactions using the two types of ligands35, we envisioned that using NHC ligand, instead of PPh3, in the current reaction might lead to predominantly branched product. Indeed, with imidazolium salts IPr·HCl (L1), the precursor to corresponding NHC ligand on deprotonation, branched product 3a was generated in 95% yield with a B/L ratio of 96/4 in the reaction of 1a with 2a (entry 1, Table 1). Since NHC has a better performance than PPh3, leading to highly selective formation of branched product in the reaction, only NHC ligands were used in the subsequent reaction condition screening.
The diastereomeric ratio (dr) for the reaction of 1a and 2a in the presence of an NHC ligand, determined by the ratio of syn-3/anti-3, was modest, 20/80 (entry 1, Table 1). To increase the diastereoselectivity of this reaction, ketones of substituted 1a were tested. Reaction of butyrophenone 1b did not show any increase in diastereoselectivity (entry 2, Table 1). Surprisingly, when β-methyl butyrophenone 1c was use as the substrate, the diastereoselectivity was reversed, and the syn-isomer became the major product with a syn/anti ratio of 85/15 (entry 3 versus entry 1, Table 1).
Next, we examined the effect of the structure of the NHC ligand on the diastereoselectivity of this reaction. Using an NHC ligand with a less sterically bulky aryl substituent, derived from L2, or 1,3-di-tert-butyl NHC, derived from L3, led to predominant linear product in low yield (entries 4 and 5, Table 1). A lower yield was obtained if the reaction was run at lower temperature (entry 6 versus entry 3, Table 1). Using an NHC ligand derived from S-IPr·HCl (L4), a dihydrogenated form of IPr·HCl (L1), high yields with excellent regio and diastereoselectivities were achieved (entry 7, Table 1; see Supplementary Table 5 for details). These results show that the structure of the NHC ligand is critical in controlling the regio and diastereoselectivities of the reaction.
In addition to ketones, some other carbonyl compounds were also examined in our catalyst system. Reaction of butyryl trimethylsilane 1ba led to 3ba in 72% yield with excellent regio and diastereoselectivities (entry 8, Table 1). Similar yield and B/L ratio, yet much lower dr were observed in the reaction of β-methyl substituted butyryl trimethylsilane 1ca (entry 9, Table 1). No desired product was observed when N-acyl pyrrole 1bb (entry 10, Table 1) or ethyl acetate (not shown in the table) was used as substrate.
We also investigated preliminarily the enantioselectivity of reactions of 1b and 1c using chiral NHCs. So far, only poor enantioselectivity with lower regio and diastereoselectivities has been achieved (see Supplementary Tables 7 and 8 for details). Realizing highly asymmetric induction in this type of reaction is the task of further investigations.
Using β-substituted ketones to construct three stereocentres
The structural motif of three contiguous stereocentres in an acyclic system can be found in a wide range of natural products, pharmaceuticals and synthetic building blocks36,37,38. In view of above results and following investigations on the mechanism (vide infra, Mechanistic investigations), we reasoned that highly selective formation of three contiguous stereocentres in this reaction might be possible if we introduce a chiral centre at the β position of 1c by changing one methyl at the β position to other groups. Following this thought, reactions of ketones 1 with different β-substituents were carried out. While using ketones 1d and 1e with, respectively, nhexyl and ethyl at the β position led to very low diastereoselectivities (entries 1 and 2, Table 2), using β-phenyl substituted ketone 1f results in 98% branched product (syn, anti)-3f with 86/14 dr (entry 3, Table 2; see Supplementary Table 6 for details). These results seemed to indicate that unsaturated groups at the β position are necessary to achieve high dr, presumably due to some favourable interaction between the unsaturated group at the β position of ketone and the phenyl on allyl39,40,41. It could be deduced that high stereoselectivity might also be obtained if other unsaturated groups were installed at the β position of ketone. Indeed, higher stereoselectivity was observed in reactions of β-alkenyl ketone 1g (ref. 42) and 1h, and β-alkynyl ketone 1i (ref. 43), with dr of 77/23, 86/14 and 93/7, respectively (entries 4–6 versus entry 1, Table 2). Note, also the high yields and regioselectivity for 3g (92%, B/L=93/7) and 3i (99%, B/L=95/5), and somewhat lower yield and regioselectivity of reaction of Z-type β-alkenyl ketone 1h, compared with those of reaction of E-type β-alkenyl ketone 1g.
Substrate scope
With the optimized reaction conditions, the compatibility of both ketone substrates and allyl reagents in the reaction was studied. As depicted in Table 3, the reaction is efficient for a wide range of β-substituted ketones, 1f–1l, affording the branched products 3f–3l bearing three contiguous stereocentres in nearly quantitative yields, with excellent regio and diastereoselectivities. Specifically, not only β-phenyl ketones (1f and 1j), but also β-alkynyl ketones with a variety of terminal substituents on alkynyl (1i, 1k and 1l) are suitable substrates for this reaction. Notably, ketones with sterically bulky substituents also react smoothly to produce the corresponding allylated product 3y in high yield with high diastereoselectivity, despite the somewhat lower B/L ratio of 85/15. Regarding the allyl reagents, we first examined mono-aryl substituted allyl reagents with both electron-withdrawing and donating substituents. With β-triisopropylsilylacetylenyl ketone 1l as nucleophile, allylated product 3l–3v were obtained in excellent yields (> 90%) with excellent regio and diastereoselectivities (B/L>90/10 and dr>93/7). Using β-methyl butyrophenone 1c as nucleophile, equally good yields and selectivities were obtained for 3ca–3ce. We also found that furanyl- and naphthyl-substituted allyl reagents are compatible in the reaction, delivering 3w and 3x. Note that the structures of one enantiomer of each of (±)-syn-3c and (±)-(syn, anti)-3f have been determined by X-ray crystallography and are shown in Table 3.
Applications
To demonstrate the usefulness of this protocol, gram-scale reactions were carried out. Under optimal conditions, reaction of 1,6-enyne 1i can be scaled up, affording 1.34 g of product 3i, with almost no decrease in yield and selectivities (Fig. 2a). Our protocol can be used to allylate the C16 position of estrone 3-methyl ether 6, providing product 7 in 96% yield with 84/16 B/L and 91/9 dr (Fig. 2b). Since optically active β-substituted ketones are easily available44,45,46,47,48,49, the capacity of this reaction for chirality transfer from optically active substrates was explored (Fig. 2cd). Products (3R, 4S, 5S)-3j and (3R, 4S, 5S)-3l were obtained in excellent yields with excellent regio and diastereoselectivities without loss of optical activity when optical active ketones (S)-1j (ref. 47) and (S)-1l (ref. 48) were used.
1,6-Enynes are important and versatile synthetic intermediates50,51. Thus, further transformations of the 1,6-enyne product 3i, generated by the titled Pd/NHC-catalysed allylic substitution, were studied. Subjecting 3i to Pauson–Khand reaction conditions leads to bicyclo[3.3.0]octane 8, which has been found to be a core skeleton in many natural products52,53, in 70% yield with excellent stereoselectivity (Fig. 3a). Also, desilylation of 3i with tetra-n-butylammonium fluoride, followed by intramolecular cyclization in the presence of ethylene and Grubbs II catalyst, yields tetra-substituted cyclopentene (2S, 3S, 4R)-10 with three contiguous chiral centres in 83% yield without loss of optical activity (Fig. 3b). The 1-vinyl cyclopentene framework in 10 is a useful building block in the synthesis of many complex molecules54,55,56,57.
Mechanistic investigations
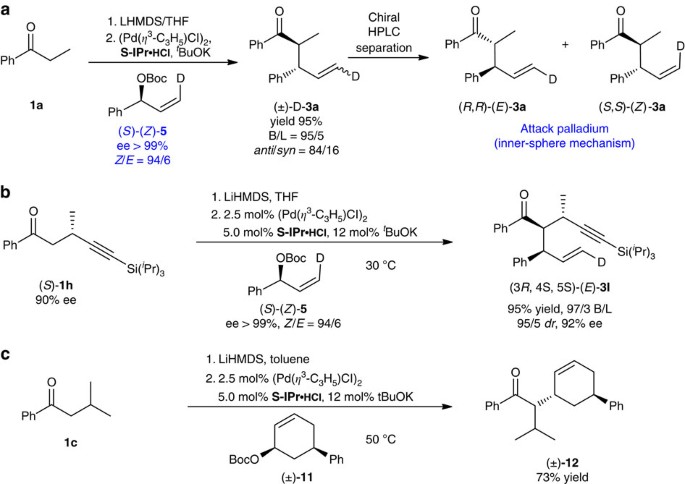
To shed some light on the reaction mechanism, deuterium-labelled and cyclic allyl reagents were used as probes. According to the known stereochemistry Pd-catalysed allylic substitution1,2,3, (S)-(Z)-3 and (R)-(E)-3 will be formed if the reaction of deuterium-labelled, optically active, allyl ester (S)-(Z)-5 proceeds via an inner-sphere mechanism, while (R)-(Z)-3 and (S)-(E)-3 will be afforded when the outer-sphere mechanism is at work (Fig. 4).
When (S)-(Z)-5 was subjected to reaction with ketone 1a (Fig. 5a)33, only (R, R)-(E)-3a and (S, S)-(Z)-3a were obtained after chiral HPLC separation, which clearly suggests that the reaction proceeds via an inner-sphere mechanism. Similarly, the reaction of (S)-(Z)-5 with β-substituted ketones (S)-1h yielding (3R, 4S, 5S)-(E)-3i (Fig. 5b), also supports that the reaction proceeds via an inner-sphere mechanism. In addition, the fact that the reaction of cis-disubstituted cyclohexene 11 afforded trans-product 12 in 73% isolated yield as a single diastereomer (determined by 1H NMR spectroscopy), again, confirms the inner-sphere mechanism (Fig. 5c)7,10.
(a) (R, R)-(E)-3a And (S, S)-(Z)-3a were afforded by the reaction of (S)-(Z)-5 with ketone 1a, indicating that the reaction proceeds via the inner-sphere mechanism. (b) (3R, 4S, 5S)-(E)-3i was the only product in the reaction of (S)-(Z)-5 supports an inner-sphere mechanism. (c) Inversion of the stereocentre at the allyl position of cyclohexene 11 in the reaction confirms also the inner-sphere mechanism.
To further understand the reaction mechanism, DFT calculations were carried out on both inner-sphere and outer-sphere pathways (Fig. 6). The energy reference is set as separated reactants (that is, lithium enolate and allyl-Pd complex). In outer-sphere pathways, the transition states leading to linear product (TS-outer-linear) and branched product (TS-outer-branched) were calculated to be, respectively, 33.9 and 41.3 kcal mol−1 above the separated reactants. These very high-reaction barriers suggest that the outer-sphere mechanism is unlikely to be operative. In addition, if an outer-sphere pathway were followed, linear product would be predicted to be formed exclusively, which contradicts experimental observations. In inner-sphere pathways, lithium enolate first reacts, exothermically, with allyl-Pd complex to yield allyl-Pd enolate (IM1 or IM2) and tBuOLi; then, [3,3’] reductive elimination takes place in the allyl-Pd enolate. The transition state for the branched product (TS-inner-branched) was computed to be 14.9 kcal mol−1 above separated reactants, much lower in energy than TS-inner-linear and the two transition states in outer-sphere pathways. These calculations suggest that the branched product is the kinetically favourable one and should be formed as the major product in experiment through an inner-sphere mechanism. We note here previous theoretical work on the inner- and outer-sphere mechanisms of allylic alkylation of lactones58.
Free energies in solvent ΔGsol (in kcal mol−1) are computed at ωB97XD/def2-TZVP/SMD//ωB97XD/SDDAll level of theory. (More detailed calculations on E-enolate and η1-allyl-Pd intermediates can be found in Supplementary Figures 69 and 71). These calculations suggest that the inner-sphere pathway leading to branched product is the most favourable pathway, in agreement with experiment.
To explain the stereochemistry of the reaction, possible conformations of the favourable transition state (TS-inner-branched) of the inner-sphere pathway were explored by DFT calculations (Fig. 7). We find that for the reaction of ketone 1b, the seven-membered ring transition state with a chair conformation is favoured over the boat transition state by 1.4 kcal mol−1. The favourable chair transition state leads to anti product, in agreement with experiment. On the other hand, the boat transition state was predicted to be lower in energy than the chair transition state by 2.0 kcal mol−1 for the reaction of ketone 1c, suggesting that syn product should be the major one. The reversal of diastereoselectivity, predicted by calculations and observed in experiments, on going from reaction of 1b to 1c, is probably associated with 1,3-diaxial interaction in the chair transition state. The steric encumbrance in the chair transition state for 1c, arising from the phenyl on the allyl and one methyl at the β position of the enolate, might destabilize the chair transition state, making it higher in energy than the boat transitions state. However, such, presumably unfavourable, 1,3-diaxial interaction is absent in the chair transition state for 1b.
For substrate 1b, the chair transition state leading to anti product is favoured, whereas for substrate 1c, the boat transition state leading to syn product is favoured. Detailed analysis and calculations on possible conformations can be found in Supplementary Figures 70–73. Numbers at the bottom of each structure are relative free energies ΔΔGsol (in kcal mol−1), computed at ωB97XD/def2-TZVP/SMD//ωB97XD/SDDAll level of theory. In the pictures in the central column, some atoms of the bulky substituents on NHC are omitted for clarity.
In summary, a novel, simple and effective Pd/NHC-catalysed protocol has been developed to produce acyclic α-allylated ketones bearing three contiguous stereocentres with excellent regio and diastereoselectivities, starting with readily available ketones and allyl reagents. This reaction features facile yet highly efficient Pd catalysis, the use of commercially available NHC ligands and wide substrate scope. It was found that substituents on the NHC and β-substituents on ketones have a critical impact on the stereochemistry of the reaction. The synthetic applications of the methodology have also been examined preliminarily. The products from current protocol are likely to be useful in the synthesis of more complex molecules. Mechanistic investigations using stereo-probing allyl reagents and DFT calculations suggest that the reaction proceeds via an inner-sphere mechanism. DFT calculations also showed that the diastereoselectivity of this reaction is highly dependent on the β-substituent on ketone.
Methods
Materials
For 1H and 13C NMR spectra of the compounds in this article, see Supplementary Figs 1–66. For X-ray analysis data of 3c, 3f, 7 and 8, see Supplementary Tables 1–4. Details of DFT calculations see Supplementary Figs 67–73 and Supplementary Information Computational methods. For coordinates and energies of calculated structures, see Supplementary Data 1.
General
Commercially available reagents were used without further purification. Solvents were purified before use according to the standard methods. Unless otherwise noted, all reactions were carried out under an atmosphere of argon and flame-dried glassware with standard vacuum-line techniques. NMR spectra are recorded at room temperature on 400 MHz Varian-400, 400M Agilent-400 or 300 MHz Bruker AM-300 NMR spectrometers. The chemical shifts for 1H NMR are reported in p.p.m. from tetramethylsilane with the solvent resonance as the internal standard (7.26 p.p.m. for CHCl3). Data are reported as follows: chemical shift, multiplicity (s=singlet, d=doublet, t=triplet, q=quartet, sept=septet, bs=broad singlet, m=multiplet), coupling constants (Hz) and integration. Chemical shifts are reported in p.p.m. from tetramethylsilane with the solvent resonance as the internal standard (CDCl3: 77.15 p.p.m.). Infra-red spectra were measured in cm−1. HRMS were carried out on the Finnigan MAT 8430 spectrometer. Thin-layer chromatography was performed on pre-coated glassback plates and visualized with ultraviolet light at 254 nm. Flash column chromatography was performed on silica gel.
General procedure for the palladium-catalysed allylic alkylation
A dry Schlenk tube was flame-dried and flushed with Argon. Ketone 1 (0.4 mmol) and toluene (2.0 ml) were added into the dry Schlenk tube. LiHMDS(1.0 M in THF, 0.4 ml, 0.4 mmol) were added at 0 °C and stirred at room temperature for 30 min. In a separated flask, [Pd(η3-C3H5)Cl]2 (1.9 mg, 0.005 mmol), S-IPr·HCl (4.2 mg, 0.005 mmol) and toluene (1.0 ml) were mixed, followed by addition of t-BuOK (1.0 M in THF, 25 μl, 0.025 mmol) at 0 °C. The resulting mixture was stirred at room temperature for 30 min, then added to the ketone solution. The allylic substrate 2 (0.2 mmol) and toluene (1.0 ml) was then added and the mixture was stirred at corresponding temperature. After the reaction was complete, the reaction mixture was quenched by H2O (0.5 ml). The solution was dried (anhydrous Na2SO4) and then filtered through a 0.5-inch plug of silica gel (eluting with EtOAc) to remove the solid. The crude reaction mixture was concentrated under reduced pressure. CDCl3 (0.7–0.8 ml) was added to dissolve the crude reaction mixture, and mesitylene (23 μl) was added as an internal standard. The regio and diastereoselectivity was then determined by 1H NMR spectroscopy. After this analysis, the crude reaction mixture was purified by flash column silica gel chromatography (eluting with petroleum ether/toluene 1/1 or petroleum ether/ethyl acetate 10/1) to afford the product 3. For additional procedures see Supplementary Methods.
Data availability
The authors declare that the data supporting the findings of this study are available within the article (and Supplementary Information files), and also are available from the corresponding author on request.
Additional information
Accession codes: The X-ray crystallographic coordinates for the structures reported in this article have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition number CCDC 1457162 (for 3c), 1457163 (for 3f), 1457165 (for 7) and 1457166 (for 8). These data can be obtained free of charge from the Cambridge Crystallographic Data Centre via http://www.ccdc.cam.ac.uk/data_request/cif.
How to cite this article: Bai, D.-C. et al. Palladium/N-heterocyclic carbene catalysed regio and diastereoselective reaction of ketones with allyl reagents via inner-sphere mechanism. Nat. Commun. 7:11806 doi: 10.1038/ncomms11806 (2016).
References
Trost, B. M. & Crawley, M. L. Asymmetric transition-metal-catalyzed allylic alkylations: applications in total synthesis. Chem. Rev. 103, 2921–2944 (2003).
Lu, Z. & Ma, S. Metal-catalyzed enantioselective allylation in asymmetric synthesis. Angew. Chem. Int. Ed. 47, 258–297 (2008).
Ding, C.-H. & Hou, X.-L. Transition metal-catalyzed asymmetric allylation. Top Organomet. Chem. 36, 247–285 (2011).
Oliver, S. & Evens, P. A. Transition-metal-catalyzed allylic substitution reactions: stereoselective construction of α- and β-substituted carbonyl compounds. Synthesis 45, 3179–3198 (2013).
You, S.-L., Zhu, X.-Z., Luo, Y.-M., Hou, X.-L. & Dai, L.-X. Highly regio- and enantioselective Pd-catalyzed allylic alkylation and amination of monosubstituted allylic acetates with novel ferrocene P,N-ligands. J. Am. Chem. Soc. 123, 7471–7472 (2001).
Weiß, T. D., Helmchen, G. & Kazmaier, U. Synthesis of amino acid derivatives via enantio- and diastereoselective Pd-catalyzed allylic substitutions with a non-stabilized enolate as nucleophile. Chem. Commun. 12, 1270–1271 (2002).
Trost, B. M. & Thaisrivongs, D. A. Strategy for employing unstabilized nucleophiles in palladium-catalyzed asymmetric allylic alkylations. J. Am. Chem. Soc. 130, 14092–14093 (2008).
Zhang, K., Peng, Q., Hou, X.-L. & Wu, Y.-D. Highly enantioselective Palladium-catalyzed alkylation of acyclic amides. Angew. Chem. Int. Ed. 47, 1741–1744 (2008).
Trost, B. M., Xu, J.-Y. & Schmidt, T. Palladium-catalyzed decarboxylative asymmetric allylic alkylation of enol carbonates. J. Am. Chem. Soc. 131, 18343–18357 (2009).
Sha, S.-C., Zhang, J.-D., Carroll, P. J. & Walsh, P. J. Raising the pKa Limit of “Soft” nucleophiles in Palladium-catalyzed allylic substitutions: application of diarylmethane pronucleophiles. J. Am. Chem. Soc. 135, 17602–1760 (2013).
Mukherjee, S. & List, B. Chiral counter anions in asymmetric transition-metal catalysis: highly enantioselective Pd/Brønsted acid-catalyzed direct α-allylation of aldehydes. J. Am. Chem. Soc. 129, 11336–11337 (2007).
Jiang, G.-X. & List, B. Direct asymmetric α-allylation of aldehydes with simple allylic alcohols enabled by the concerted action of three different catalysts. Angew. Chem. Int. Ed. 50, 9471–9474 (2011).
Negishi E. Handbook of Organopalladium Chemistry For Organic Synthesis Wiley (2002).
Bäckvall, J.-E., Nordberg, R. E., Björkman, E. E. & Moberg, C. Stereochemistry of nucleophilic attack on π-allylpalladium complexes. Evidence for cis-migration of acetate from palladium to carbon. J. Chem. Soc. Chem. Commun. 943–944 (1980).
Hayashi, T., Iwamura, H., Naito, M., Matsumoto, Y. & Uozumi, Y. Catalytic asymmetric reduction of allylic esters with formic acid catalyzed by Pd-MOP complexes. J. Am. Chem. Soc. 116, 775–776 (1994).
Nakamura, H., Bao, M. & Yamamoto, Y. The Fate of Bis(η3-allyl)palladium complexes in the presence of aldehydes (or imines) and allylic chlorides: stille coupling versus allylation of aldehydes (or imines). Angew. Chem. Int. Ed. 40, 3208–3210 (2001).
Stokes, B. J., Opra, S. M. & Sigman, M. S. Palladium-catalyzed allylic cross-coupling reactions of primary and secondary homoallylic electrophiles. J. Am. Chem. Soc. 134, 11408–11411 (2012).
Li, Y.-X. et al. A Pd(0)-catalyzed direct dehydrative coupling of terminal alkynes with allylic alcohols to access 1,4-enynes. J. Am. Chem. Soc. 135, 12536–12539 (2013).
Bai, D.-C., Wang, W.-Y., Ding, C.-H. & Hou, X.-L. Kinetic resolution of unsymmetrical acyclic allyl carbonates using trimethylsilyl cyanide via Palladium-catalyzed asymmetric allylic alkylation. Synlett 26, 1510–1514 (2015).
Xiang, S.-H., Hoang, K. L. M., He, J.-X., Tan, Y.-J. & Liu, X.-W. Reversing the stereoselectivity of a palladium-catalyzed O-Glycosylation through an inner-sphere or outer-sphere pathway. Angew. Chem. Int. Ed. 54, 604–607 (2015).
Méndez, M., Cuerva, J. M., Gómez-Bengoa, E., Cárdenas, D. J. & Echavarren, A. M. Intramolecular coupling of allyl carboxylates with allylstannanes and allylsilanes: a new type of reductive elimination reaction? Chem. Eur. J. 8, 3620–3628 (2002).
Cárdenas, D. J. & Echavarren, A. M. Mechanistic aspects of C–C bond formation involving allylpalladium complexes: the role of computational studies. New J. Chem. 28, 338–347 (2004).
Luzung, M. R., Lewis, C. A. & Baran, P. S. Direct, chemoselective N-tert -prenylation of indoles by C-H functionalization. Angew. Chem. Int. Ed. 48, 7025–7029 (2009).
Perez-Rodriguez, M. et al. A DFT study of the effect of the ligands in the reductive elimination from Palladium bis(allyl) complexes. Organometallics 29, 4983–4991 (2010).
Keith, J. A. et al. The inner-sphere process in the enantioselective Tsuji allylation reaction with (S)-t-Bu-phosphinooxazoline ligands. J. Am. Chem. Soc. 129, 11876–11877 (2007).
Enquist, J. A. Jr. & Stoltz, B. M. The total synthesis of (-)-cyanthiwigin F by means of double catalytic enantioselective alkylation. Nature 453, 1228–1231 (2008).
Sherden, N. H., Behenna, D. C., Virgil, S. C. & Stoltz, B. M. Unusual allylpalladium carboxylate complexes: identification of the resting state of catalytic enantioselective decarboxylative allylic alkylation reactions of ketones. Angew. Chem. Int. Ed. 48, 6840–6843 (2009).
Zhang, P., Brozek, L. A. & Morken, J. P. Pd-catalyzed enantioselective allyl-allyl cross-coupling. J. Am. Chem. Soc. 132, 10686–10688 (2010).
Zhang, P., Le, H., Kyne, R. E. & Morken, J. P. Enantioselective construction of all-carbon quaternary centers by branch-selective Pd-catalyzed allyl–allyl cross-coupling. J. Am. Chem. Soc. 133, 9716–9719 (2011).
Brozek, L. A., Ardolino, M. J. & Morken, J. P. Diastereocontrol in asymmetric allyl–allyl cross-coupling: stereocontrolled reaction of prochiral allylboronates with prochiral allyl chlorides. J. Am. Chem. Soc. 133, 16778–16781 (2011).
Ardolino, M. J. & Morken, J. P. Congested C−C bonds by Pd-catalyzed enantioselective allyl-allyl cross-coupling, a mechanism-guided solution. J. Am. Chem. Soc. 136, 7092–7100 (2014).
Ardolino, M. J. & Morken, J. P. Construction of 1,5-enynes by stereospecific Pd-catalyzed allyl-propargyl cross-couplings. J. Am. Chem. Soc. 134, 8770–8773 (2012).
Chen, J.-P., Peng, Q., Lei, B.-L., Hou, X.-L. & Wu, Y.-D. Chemo- and regioselectivity-tunable Pd-catalyzed allylic alkylation of imines. J. Am. Chem. Soc. 133, 14180–14183 (2011).
Keith, J. A. et al. The reaction mechanism of the enantioselective Tsuji allylation: inner-sphere and outer-sphere pathways, internal rearrangements, and asymmetric C−C Bond formation. J. Am. Chem. Soc. 134, 19050–19060 (2012).
Nolan S. P. N-Heterocyclic Carbenes Wiley-VCH (2014).
Nicolaou, K. C., Vourloumis, D., Winssinger, M. & Baran, P. S. The art and science of total synthesis at the dawn of the twenty-first century. Angew. Chem. Int. Ed. 39, 44–122 (2000).
Gaich, T. & Baran, P. S. Aiming for the ideal synthesis. J. Org. Chem. 75, 4657–4673 (2010).
Newman, D. J. & Cragg, G. M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 75, 311–335 (2012).
Wheeler, S. E. Understanding substituent effects in noncovalent interactions involving aromatic rings. Acc. Chem. Res. 46, 1029–1038 (2013).
Wheeler, S. E. & Bloom, J. W. G. Toward a more complete understanding of noncovalent interactions involving aromatic rings. J. Phys. Chem. A. 118, 6133–6147 (2014).
Seguin, T. J., Lu, T.-X. & Wheeler, S. E. Enantioselectivity in catalytic asymmetric fischer indolizations hinges on the competition of π-stacking and CH/π interactions. Org. Lett. 17, 3066–3069 (2015).
Roscales, S., Rincón, Ángela., Buxaderas, E. & Csákÿ, A. G. Trifluoroacetic anhydride-catalyzed conjugate addition of boronic acids to α,β-unsaturated ketones. Tetrahedron. Lett. 53, 4721–4724 (2012).
Chong, J. M., Shen, L. X. & Taylor, N. J. Asymmetric conjugate addition of alkynylboronates to enones. J. Am. Chem. Soc. 122, 1822–1823 (2000).
Krause, N. & Hoffmann-Roder, A. Recent advances in catalytic enantioselective Michael additions. Synthesis 2, 171–196 (2001).
Hayashi, T. & Yamasaki, K. Rhodium-catalyzed asymmetric 1,4-addition and its related asymmetric reactions. Chem. Rev. 103, 2829–2844 (2003).
Tsogoeva, S. B. Recent advances in asymmetric organocatalytic 1,4-conjugate additions. Eur. J. Org. Chem. 11, 1701–1716 (2007).
Endo, K., Ogawa, M. & Shibata, T. Multinuclear catalyst for copper-catalyzed asymmetric conjugate addition of organozinc reagents. Angew. Chem. Int. Ed. 49, 2410–2413 (2010).
Nishimura, T., Guo, X.-X., Uchiyama, N., Katoh, T. & Hayashi, T. Steric tuning of silylacetylenes and chiral phosphine ligands for Rhodium-catalyzed asymmetric conjugate alkynylation of enones. J. Am.Chem. Soc. 130, 1576–1577 (2008).
Guo, X. T., Shen, J., Sha, F. & Wu, X.-Y. Highly enantioselective Michael addition of nitroalkanes to enones and its application in syntheses of (R)-Baclofen and (R)-Phenibut. Synthesis 47, 2063–2072 (2015).
Diver, S. T. & Giessert, A. J. Enyne metathesis (enyne bond reorganization). Chem. Rev. 104, 1317–1382 (2004).
Blanco-Urgoiti, J., Añorbe, L., Pérez-Serrano, L., Domínguez, G. & Pérez-Castells, J. The Pauson–Khand reaction, a powerful synthetic tool for the synthesis of complex molecules. Chem. Soc. Rev. 33, 32–42 (2004).
Mukai, C., Kim, J. S., Sonobe, H. & Hanaoka, M. Stereocomplementary construction of optically active bicyclo[4.3.0]nonenone derivatives. J. Org. Chem. 64, 6822–6832 (1999).
Hiroi, K., Watanabe, T., Kawagishi, R. & Abe, I. Catalytic use of chiral phosphine ligands in asymmetric Pauson–Khand reactions. Tetrahedron. Asymmetry. 11, 797–808 (2000).
Dolhem, F., Lièvre, C. & Demailly, G. Synthesis of carbohydrate-derived enynes and subsequent metathesis to yield polyhydroxylated 1-vinylcycloalkenes. Eur. J. Org. Chem. 12, 2336–2342 (2003).
Funel, J.-A. & Prunet, J. Enyne versus diene RCM in the synthesis of cyclopentene derivatives toward the A ring of FR182877. J. Org. Chem. 69, 4555–4558 (2004).
Xue, Z. J., Chen, P., Peng, S. Y. & Li, Y. C. Synthesis and determination of absolute configuration of tetracetate 4a-carba-D-xylofuranoside. Tetrahedron 62, 199–204 (2006).
Krishna, P. R. & Alivelu, M. Stereoselective synthesis of aminocyclitol moieties of trehazolin and trehalostatin via enyne metathesis protocol. Tetrahedron Lett. 51, 6265–6267 (2010).
Patil, M. & Thiel, W. Origin of selectivity of Tsuji–Trost allylic alkylation of lactones: highly ordered transition states with lithium-containing enolates. Chem. Eur. J. 18, 10408–10418 (2012).
Acknowledgements
This paper is dedicated to Professor Henry N. C. Wong on the occasion of his 65th birthday. Financial support by the Major Basic Research Development Program (2011CB808706), NSFC (21272251, 21532010, 21421091), the NSFC and the RGC Hong Kong Joint Research Scheme (21361162001), the Chinese Academy of Sciences, the Technology Commission of Shanghai Municipality and the Croucher Foundation of Hong Kong is gratefully acknowledged. The computing facilities used in this work at Cornell were supported by EFree (an Energy Frontier Research Center funded by the U.S. Department of Energy (Award No. DESC0001057 at Cornell, subcontract to Carnegie Institution of Washington)). We thank Professor Roald Hoffmann at Cornell University for language revision.
Author information
Authors and Affiliations
Contributions
D.-C.B. initiated the project, designed and completed the experimental works and product characterizations; F.-L.Y., W.-Y.W., H.L., Q.-R.L. did some experiments; D.-C.B. did some analysis of products; C.-H.D. designed some experiments and wrote the manuscript with others; B.C. completed the DFT calculations and wrote the calculation part of the manuscript; X.-L.H. designed and directed the project and wrote the manuscript. All authors joined to discussions.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Figures 1-73, Supplementary Tables 1-8, Supplementary Methods and Supplementary References (PDF 6890 kb)
Supplementary Data 1
Energies and coordinates of calculated structures (PDF 2907 kb)
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Bai, DC., Yu, FL., Wang, WY. et al. Palladium/N-heterocyclic carbene catalysed regio and diastereoselective reaction of ketones with allyl reagents via inner-sphere mechanism. Nat Commun 7, 11806 (2016). https://doi.org/10.1038/ncomms11806
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/ncomms11806
This article is cited by
-
Palladium-catalysed branch- and enantioselective allylic C–H alkylation of α-alkenes
Nature Synthesis (2022)
-
Regio- and enantioselective umpolung gem-difluoroallylation of hydrazones via palladium catalysis enabled by N-heterocyclic carbene ligand
Nature Communications (2021)
-
Photocatalytic enantioselective α-aminoalkylation of acyclic imine derivatives by a chiral copper catalyst
Nature Communications (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.