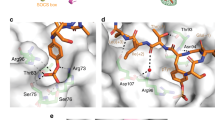
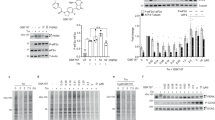
Abstract
p90 ribosomal protein S6 kinases (RSKs) integrate upstream signals through two catalytic domains. Autophosphorylation of Ser386 by the regulatory C-terminal kinase domain (CTD) is thought to be essential for activation of the N-terminal kinase domain (NTD), which phosphorylates multiple downstream targets1. We recently reported fmk, an irreversible inhibitor of the CTD of RSK1 and RSK22. Here we describe fmk-pa, a propargylamine variant that has improved cellular potency and a 'clickable' tag for assessing the extent and selectivity of covalent RSK modification. Copper-catalyzed conjugation of an azidoalkyl reporter (the click reaction) revealed that fmk-pa achieves selective and saturable modification of endogenous RSK1 and RSK2 in mammalian cells. Saturating concentrations of fmk-pa inhibited Ser386 phosphorylation and downstream signaling in response to phorbol ester stimulation, but had no effect on RSK activation by lipopolysaccharide. RSK autoactivation by the CTD is therefore context dependent, which suggests that NTD and CTD inhibitors should have distinct physiological effects.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Hauge, C. & Frodin, M. RSK and MSK in MAP kinase signalling. J. Cell Sci. 119, 3021–3023 (2006).
Cohen, M.S., Zhang, C., Shokat, K.M. & Taunton, J. Structural bioinformatics-based design of selective, irreversible kinase inhibitors. Science 308, 1318–1321 (2005).
Smith, J.A., Poteet-Smith, C.E., Malarkey, K. & Sturgill, T.W. Identification of an extracellular signal-regulated kinase (ERK) docking site in ribosomal S6 kinase, a sequence critical for activation by ERK in vivo. J. Biol. Chem. 274, 2893–2898 (1999).
Gavin, A.C. & Nebreda, A.R.A. MAP kinase docking site is required for phosphorylation and activation of p90(rsk)/MAPKAP kinase-1. Curr. Biol. 9, 281–284 (1999).
Frodin, M., Jensen, C.J., Merienne, K. & Gammeltoft, S. A phosphoserine-regulated docking site in the protein kinase RSK2 that recruits and activates PDK1. EMBO J. 19, 2924–2934 (2000).
Jensen, C.J. et al. 90-kDa ribosomal S6 kinase is phosphorylated and activated by 3-phosphoinositide-dependent protein kinase-1. J. Biol. Chem. 274, 27168–27176 (1999).
Richards, S.A., Fu, J., Romanelli, A., Shimamura, A. & Blenis, J. Ribosomal S6 kinase 1 (RSK1) activation requires signals dependent on and independent of the MAP kinase ERK. Curr. Biol. 9, 810–820 (1999).
Fisher, T.L. & Blenis, J. Evidence for two catalytically active kinase domains in pp90rsk. Mol. Cell. Biol. 16, 1212–1219 (1996).
Dalby, K.N., Morrice, N., Caudwell, F.B., Avruch, J. & Cohen, P. Identification of regulatory phosphorylation sites in mitogen-activated protein kinase (MAPK)-activated protein kinase-1a/p90rsk that are inducible by MAPK. J. Biol. Chem. 273, 1496–1505 (1998).
Vik, T.A. & Ryder, J.W. Identification of serine 380 as the major site of autophosphorylation of Xenopus pp90rsk. Biochem. Biophys. Res. Commun. 235, 398–402 (1997).
Bjorbaek, C., Zhao, Y. & Moller, D.E. Divergent functional roles for p90rsk kinase domains. J. Biol. Chem. 270, 18848–18852 (1995).
Chrestensen, C.A. & Sturgill, T.W. Characterization of the p90 ribosomal S6 kinase 2 carboxyl-terminal domain as a protein kinase. J. Biol. Chem. 277, 27733–27741 (2002).
Vocadlo, D.J. & Bertozzi, C.R. A strategy for functional proteomic analysis of glycosidase activity from cell lysates. Angew. Chem. Int. Edn Engl. 43, 5338–5342 (2004).
Speers, A.E., Adam, G.C. & Cravatt, B.F. Activity-based protein profiling in vivo using a copper(I)-catalyzed azide-alkyne [3 + 2] cycloaddition. J. Am. Chem. Soc. 125, 4686–4687 (2003).
Speers, A.E. & Cravatt, B.F. Profiling enzyme activities in vivo using click chemistry methods. Chem. Biol. 11, 535–546 (2004).
Ovaa, H. et al. Chemistry in living cells: detection of active proteasomes by a two-step labeling strategy. Angew. Chem. Int. Edn Engl. 42, 3626–3629 (2003).
Alexander, J.P. & Cravatt, B.F. Mechanism of carbamate inactivation of FAAH: implications for the design of covalent inhibitors and in vivo functional probes for enzymes. Chem. Biol. 12, 1179–1187 (2005).
Evans, M.J., Saghatelian, A., Sorensen, E.J. & Cravatt, B.F. Target discovery in small-molecule cell-based screens by in situ proteome reactivity profiling. Nat. Biotechnol. 23, 1303–1307 (2005).
Ruvinsky, I. et al. Ribosomal protein S6 phosphorylation is a determinant of cell size and glucose homeostasis. Genes Dev. 19, 2199–2211 (2005).
Hay, N. & Sonenberg, N. Upstream and downstream of mTOR. Genes Dev. 18, 1926–1945 (2004).
Pende, M. et al. S6K1(−/−)/S6K2(−/−) mice exhibit perinatal lethality and rapamycin-sensitive 5′-terminal oligopyrimidine mRNA translation and reveal a mitogen-activated protein kinase-dependent S6 kinase pathway. Mol. Cell. Biol. 24, 3112–3124 (2004).
Leighton, I.A., Dalby, K.N., Caudwell, F.B., Cohen, P.T. & Cohen, P. Comparison of the specificities of p70 S6 kinase and MAPKAP kinase-1 identifies a relatively specific substrate for p70 S6 kinase: the N-terminal kinase domain of MAPKAP kinase-1 is essential for peptide phosphorylation. FEBS Lett. 375, 289–293 (1995).
Wang, X. et al. Regulation of elongation factor 2 kinase by p90(RSK1) and p70 S6 kinase. EMBO J. 20, 4370–4379 (2001).
Shahbazian, D. et al. The mTOR/PI3K and MAPK pathways converge on eIF4B to control its phosphorylation and activity. EMBO J. 25, 2781–2791 (2006).
Sapkota, G.P . et al. BI-D1870 is a specific inhibitor of the p90 RSK (ribosomal S6 kinase) isoforms in vitro and in vivo. Biochem. J. 401, 29–38 (2007).
Smith, J.A . et al. Identification of the first specific inhibitor of p90 ribosomal S6 kinase (RSK) reveals an unexpected role for RSK in cancer cell proliferation. Cancer. Res. 65, 1027–1034 (2005).
Itoh, S. et al. Role of p90 ribosomal S6 kinase-mediated prorenin-converting enzyme in ischemic and diabetic myocardium. Circulation 113, 1787–1798 (2006).
Maekawa, N. et al. Inhibiting p90 ribosomal S6 kinase prevents (Na+)-H+ exchanger-mediated cardiac ischemia-reperfusion injury. Circulation 113, 2516–2523 (2006).
Chan, T.R., Hilgraf, R., Sharpless, K.B. & Fokin, V.V. Polytriazoles as copper(I)-stabilizing ligands in catalysis. Org. Lett. 6, 2853–2855 (2004).
Stamm, L.M. et al. Mycobacterium marinum escapes from phagosomes and is propelled by actin-based motility. J. Exp. Med. 198, 1361–1368 (2003).
Acknowledgements
This work was supported by the US National Institutes of Health (GM71434) and a University of California at San Francisco President's Dissertation-Year Fellowship to M.S.C. We thank M. Pak and E. Brown (University of California at San Francisco) for the primary mouse macrophages. We thank H. Luecke, M. Simon and members of the Taunton laboratory for many helpful discussions and for critical reading of the manuscript.
Author information
Authors and Affiliations
Contributions
J.T. and M.S.C. designed the experiments; M.S.C. and H.H. executed the experiments; J.T. and M.S.C. analyzed the data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Inhibition of PMA-stimulated RSK Ser386 phosphorylation by fmk. (PDF 104 kb)
Supplementary Fig. 2
fmk-pa covalently modifies RSK1 and RSK2 in intact cells as determined by click chemistry. (PDF 130 kb)
Supplementary Fig. 3
Covalent modification of RSK by fmk-pa is unaffected by serum in the incubation medium, whereas covalent modification by fmk-BODIPY is significantly decreased. (PDF 333 kb)
Supplementary Fig. 4
fmk-pa does not inhibit S6K phosphorylation at Thr389, an essential activation site. (PDF 124 kb)
Supplementary Fig. 5
Inhibition of rpS6 Ser235/236 phosphorylation by rapamycin and fmk-pa. (PDF 189 kb)
Supplementary Table 1
Half-maximal inhibitory concentrations (IC50s) for fmk, fmk-BODIPY and fmk-pa against the kinase activity of RSK2 CTD. (PDF 116 kb)
Supplementary Methods
Synthetic schemes and procedures. (PDF 332 kb)
Rights and permissions
About this article
Cite this article
Cohen, M., Hadjivassiliou, H. & Taunton, J. A clickable inhibitor reveals context-dependent autoactivation of p90 RSK. Nat Chem Biol 3, 156–160 (2007). https://doi.org/10.1038/nchembio859
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio859
This article is cited by
-
TRIPODD: a Novel Fluorescence Imaging Platform for In Situ Quantification of Drug Distribution and Therapeutic Response
Molecular Imaging and Biology (2021)
-
Kinase-targeted cancer therapies: progress, challenges and future directions
Molecular Cancer (2018)
-
Quantitating drug-target engagement in single cells in vitro and in vivo
Nature Chemical Biology (2017)
-
Proteogenomic convergence for understanding cancer pathways and networks
Clinical Proteomics (2014)
-
A road map to evaluate the proteome-wide selectivity of covalent kinase inhibitors
Nature Chemical Biology (2014)