Abstract
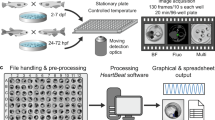
To increase the facility and throughput of scoring phenotypic traits in embryonic zebrafish, we developed an automated micro-well assay for heart rate using automated fluorescence microscopy of transgenic embryos expressing green fluorescent protein in myocardium. The assay measures heart rates efficiently and accurately over a large linear dynamic range, and it rapidly characterizes dose dependence and kinetics of small molecule–induced changes in heart rate. This is the first high-throughput micro-well assay for organ function in an intact vertebrate.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Peterson, R.T., Link, B.A., Dowling, J.E. & Schreiber, S.L. Proc. Natl. Acad. Sci. USA 97, 12965–12969 (2000).
Peterson, R.T., Mably, J.D., Chen, J.N. & Fishman, M.C. Curr. Biol. 11, 1481–1491 (2001).
MacRae, C.A. & Peterson, R.T. Chem. Biol. 10, 901–908 (2003).
Shafizadeh, E., Peterson, R.T. & Lin, S. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 138, 245–249 (2004).
Chan, J. & Serluca, F.C. Methods Cell Biol. 76, 475–487 (2004).
Peterson, R.T. et al. Nat. Biotechnol. 22, 595–599 (2004).
Zon, L.I. & Peterson, R.T. Nat. Rev. Drug Discov. 4, 35–44 (2005).
Milan, D.J., Peterson, T.A., Ruskin, J.N., Peterson, R.T. & MacRae, C.A. Circulation 107, 1355–1358 (2003).
Antkiewicz, D.S., Burns, C.G., Carney, S.A., Peterson, R.E. & Heideman, W. Toxicol. Sci. 84, 368–377 (2005).
Hill, A.J., Teraoka, H., Heideman, W. & Peterson, R.E. Toxicol. Sci. 86, 6–19 (2005).
Huang, C.J., Tu, C.T., Hsiao, C.D., Hsieh, F.J. & Tsai, H.J. Dev. Dyn. 228, 30–40 (2003).
Hardman, J. & Limbird, L. (eds.). Goodman and Gilman's Pharmacological Basis of Therapeutics (MaGraw-Hill Companies Incorporated, New York, 2001).
Stainier, D.Y. et al. Development 123, 285–292 (1996).
Chen, J.N. et al. Development 123, 293–302 (1996).
Sehnert, A.J. & Stainier, D.Y. Trends Genet. 18, 491–494 (2002).
Acknowledgements
We thank C. Felts for assistance with the automated microscope and Metamorph (Molecular Devices) software, D. Gilmour for providing the GFP expression plasmid pG1 and R. Peterson for use of the automated microscope. This work was funded by the US National Institutes of Health (NIH) grant 5T32HL07208-26 to Massachusetts General Hospital. C.G.B. was funded by National Institutes on Aging/NIH grant 1K01AG023562-01 and National Institute of Neurological Disorders and Stroke/NIH grant 1R03NS050806-01.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Pre-exposing wells to fluorescent light prior to subsite examination increased assay efficiency. (PDF 51 kb)
Supplementary Table 1
Automated microscopy algorithm for acquisition and analysis of video images for measuring fluorescent heart rates of Tg(cmlc2:GFP) embryos arrayed in 96-well plates. (PDF 121 kb)
Rights and permissions
About this article
Cite this article
Burns, C., Milan, D., Grande, E. et al. High-throughput assay for small molecules that modulate zebrafish embryonic heart rate. Nat Chem Biol 1, 263–264 (2005). https://doi.org/10.1038/nchembio732
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio732
This article is cited by
-
Functional screening of congenital heart disease risk loci identifies 5 genes essential for heart development in zebrafish
Cellular and Molecular Life Sciences (2023)
-
Intrinsic myocardial defects underlie an Rbfox-deficient zebrafish model of hypoplastic left heart syndrome
Nature Communications (2022)
-
Zebrafish disease models in drug discovery: from preclinical modelling to clinical trials
Nature Reviews Drug Discovery (2021)
-
Automated high-throughput heartbeat quantification in medaka and zebrafish embryos under physiological conditions
Scientific Reports (2020)
-
Autophagy Activation in Zebrafish Heart Regeneration
Scientific Reports (2020)