Abstract
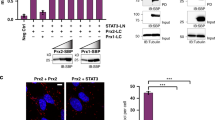
Hydrogen peroxide (H2O2) acts as a signaling messenger by oxidatively modifying distinct cysteinyl thiols in distinct target proteins. However, it remains unclear how redox-regulated proteins, which often have low intrinsic reactivity towards H2O2 (kapp ∼1–10 M−1 s−1), can be specifically and efficiently oxidized by H2O2. Moreover, cellular thiol peroxidases, which are highly abundant and efficient H2O2 scavengers, should effectively eliminate virtually all of the H2O2 produced in the cell. Here, we show that the thiol peroxidase peroxiredoxin-2 (Prx2), one of the most H2O2-reactive proteins in the cell (kapp ∼107–108 M−1 s−1), acts as a H2O2 signal receptor and transmitter in transcription factor redox regulation. Prx2 forms a redox relay with the transcription factor STAT3 in which oxidative equivalents flow from Prx2 to STAT3. The redox relay generates disulfide-linked STAT3 oligomers with attenuated transcriptional activity. Cytokine-induced STAT3 signaling is accompanied by Prx2 and STAT3 oxidation and is modulated by Prx2 expression levels.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
D'Autréaux, B. & Toledano, M.B. ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat. Rev. Mol. Cell Biol. 8, 813–824 (2007).
Finkel, T. Signal transduction by reactive oxygen species. J. Cell Biol. 194, 7–15 (2011).
Winterbourn, C.C. Reconciling the chemistry and biology of reactive oxygen species. Nat. Chem. Biol. 4, 278–286 (2008).
Winterbourn, C.C. & Hampton, M.B. Thiol chemistry and specificity in redox signaling. Free Radic. Biol. Med. 45, 549–561 (2008).
Stone, J.R. & Yang, S. Hydrogen peroxide: a signaling messenger. Antioxid. Redox Signal. 8, 243–270 (2006).
Woo, H.A. et al. Inactivation of peroxiredoxin I by phosphorylation allows localized H2O2 accumulation for cell signaling. Cell 140, 517–528 (2010).
Delaunay, A., Pflieger, D., Barrault, M.B., Vinh, J. & Toledano, M.B. A thiol peroxidase is an H2O2 receptor and redox-transducer in gene activation. Cell 111, 471–481 (2002).
Gutscher, M. et al. Proximity-based protein thiol oxidation by H2O2-scavenging peroxidases. J. Biol. Chem. 284, 31532–31540 (2009).
Fomenko, D.E. et al. Thiol peroxidases mediate specific genome-wide regulation of gene expression in response to hydrogen peroxide. Proc. Natl. Acad. Sci. USA 108, 2729–2734 (2011).
Zito, E. et al. Oxidative protein folding by an endoplasmic reticulum–localized peroxiredoxin. Mol. Cell 40, 787–797 (2010).
Tavender, T.J., Springate, J.J. & Bulleid, N.J. Recycling of peroxiredoxin IV provides a novel pathway for disulphide formation in the endoplasmic reticulum. EMBO J. 29, 4185–4197 (2010).
Wei, P.C. et al. Loss of the oxidative stress sensor NPGPx compromises GRP78 chaperone activity and induces systemic disease. Mol. Cell 48, 747–759 (2012).
Jarvis, R.M., Hughes, S.M. & Ledgerwood, E.C. Peroxiredoxin 1 functions as a signal peroxidase to receive, transduce, and transmit peroxide signals in mammalian cells. Free Radic. Biol. Med. 53, 1522–1530 (2012).
Sobotta, M.C. et al. Exposing cells to H2O2: a quantitative comparison between continuous low-dose and one-time high-dose treatments. Free Radic. Biol. Med. 60, 325–335 (2013).
Lee, W. et al. Human peroxiredoxin 1 and 2 are not duplicate proteins: the unique presence of CYS83 in Prx1 underscores the structural and functional differences between Prx1 and Prx2. J. Biol. Chem. 282, 22011–22022 (2007).
Li, L. & Shaw, P.E.A. STAT3 dimer formed by inter-chain disulphide bridging during oxidative stress. Biochem. Biophys. Res. Commun. 322, 1005–1011 (2004).
Li, L., Cheung, S.H., Evans, E.L. & Shaw, P.E. Modulation of gene expression and tumor cell growth by redox modification of STAT3. Cancer Res. 70, 8222–8232 (2010).
Peskin, A.V. et al. The high reactivity of peroxiredoxin 2 with H2O2 is not reflected in its reaction with other oxidants and thiol reagents. J. Biol. Chem. 282, 11885–11892 (2007).
Ushio-Fukai, M. Localizing NADPH oxidase-derived ROS. Sci. STKE 2006, re8 (2006).
Veal, E.A., Ross, S.J., Malakasi, P., Peacock, E. & Morgan, B.A. Ybp1 is required for the hydrogen peroxide–induced oxidation of the Yap1 transcription factor. J. Biol. Chem. 278, 30896–30904 (2003).
Calvo, I.A. et al. Dissection of a redox relay: H2O2-dependent activation of the transcription factor Pap1 through the peroxidatic Tpx1-thioredoxin cycle. Cell Reports 5, 1413–1424 (2013).
Choi, M.H. et al. Regulation of PDGF signalling and vascular remodelling by peroxiredoxin II. Nature 435, 347–353 (2005).
Ng, D.C. et al. Stat3 regulates microtubules by antagonizing the depolymerization activity of stathmin. J. Cell Biol. 172, 245–257 (2006).
Wegrzyn, J. et al. Function of mitochondrial Stat3 in cellular respiration. Science 323, 793–797 (2009).
Tammineni, P. et al. The import of the transcription factor STAT3 into mitochondria depends on GRIM-19, a component of the electron transport chain. J. Biol. Chem. 288, 4723–4732 (2013).
Okazaki, S., Naganuma, A. & Kuge, S. Peroxiredoxin-mediated redox regulation of the nuclear localization of Yap1, a transcription factor in budding yeast. Antioxid. Redox Signal. 7, 327–334 (2005).
Nadeau, P.J., Charette, S.J., Toledano, M.B. & Landry, J. Disulfide bond-mediated multimerization of Ask1 and its reduction by thioredoxin-1 regulate H2O2-induced c-Jun NH2-terminal kinase activation and apoptosis. Mol. Biol. Cell 18, 3903–3913 (2007).
Putker, M. et al. Redox-dependent control of FOXO/DAF-16 by transportin-1. Mol. Cell 49, 730–742 (2013).
Myers, M.P. et al. TYK2 and JAK2 are substrates of protein-tyrosine phosphatase 1B. J. Biol. Chem. 276, 47771–47774 (2001).
Mamoon, N.M. et al. Multiple cysteine residues are implicated in janus kinase 2-mediated catalysis. Biochemistry 46, 14810–14818 (2007).
Godoy, J.R. et al. Redox atlas of the mouse. Immunohistochemical detection of glutaredoxin-, peroxiredoxin-, and thioredoxin-family proteins in various tissues of the laboratory mouse. Biochim. Biophys. Acta 1810, 2–92 (2011).
Xu, G. et al. Thioredoxin-interacting protein regulates insulin transcription through microRNA-204. Nat. Med. 19, 1141–1146 (2013).
Morgan, B., Sobotta, M.C. & Dick, T.P. Measuring EGSH and H2O2 with roGFP2-based redox probes. Free Radic. Biol. Med. 51, 1943–1951 (2011).
Wang, X., Li, X. & Li, Y. A modified Coomassie Brilliant Blue staining method at nanogram sensitivity compatible with proteomic analysis. Biotechnol. Lett. 29, 1599–1603 (2007).
Boersema, P.J., Raijmakers, R., Lemeer, S., Mohammed, S. & Heck, A.J. Multiplex peptide stable isotope dimethyl labeling for quantitative proteomics. Nat. Protoc. 4, 484–494 (2009).
Acknowledgements
T.P.D. is supported by the Deutsche Forschungsgemeinschaft (SFB 1036, SFB 938 and SPP 1710). M.C.S. was supported by a PhD fellowship from the Boehringer Ingelheim Fonds. We thank B. Morgan and R. Jarvis for critical and helpful comments on the manuscript.
Author information
Authors and Affiliations
Contributions
M.C.S. and T.P.D. conceived the project, designed the experiments, analyzed the data and wrote the manuscript. M.C.S. performed most experiments; W.L. generated and analyzed cysteine mutants; S.S. performed luciferase reporter assays; S.S. and D.T. analyzed cytokine-induced protein oxidation; M.O. performed experiments relating to the role of thioredoxin; T.R. and A.N.D.S. performed MS experiments.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Results and Supplementary Figures 1–13. (PDF 2329 kb)
Rights and permissions
About this article
Cite this article
Sobotta, M., Liou, W., Stöcker, S. et al. Peroxiredoxin-2 and STAT3 form a redox relay for H2O2 signaling. Nat Chem Biol 11, 64–70 (2015). https://doi.org/10.1038/nchembio.1695
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.1695
This article is cited by
-
Redox-stress response resistance (RRR) mediated by hyperoxidation of peroxiredoxin 2 in senescent cells
Science China Life Sciences (2023)
-
Peroxiredoxins in erythrocytes: far beyond the antioxidant role
Journal of Molecular Medicine (2023)
-
Identification of peroxiredoxin II and its related molecules as potential biomarkers of dermal mesenchymal stem cell homing using network analysis
Applied Biological Chemistry (2022)
-
The cytosolic thiol peroxidase PRXIIB is an intracellular sensor for H2O2 that regulates plant immunity through a redox relay
Nature Plants (2022)
-
Characterization of the Neospora caninum peroxiredoxin: a novel peroxidase and antioxidant enzyme
Parasitology Research (2022)